June 2013 - Target Price $0.05c
Feb 2014 - Target Price $0.12c
Oct 2014 - Target Price $0.22c
Allied Healthcare Group
Allied Medical
Coridon Pty Ltd
Celxcel
2012 Highlights
Submitted European CE mark application for marketing authorisation of CardioCel®.
Regenerative medicine franchise is seeking approval in numerous jurisdictions.
The ADAPT® platform technology has potential to make a significant impact on the global market for structural heart defect repair, estimated at over $3Bn* .
ADAPT® TEP platform technology to be used in pelvic floor reconstructions, hernia repairs, orthopaedics and as a biological scaffold to grow and deliver stem cells.
Collaborating with CSIRO to develop ADAPT® treated tissue matrices as scaffolds for the delivery of adult mesenchymal stem cells in models of heart failure.
CardioCel® continues to gain support of key cardiothoracic surgeons
Additional authorisations for CardioCel® are expected to be announced in the near future
CardioCel®’s use in CHD patients fills a high unmet clinical need
Coridon gears up for highly anticipated herpes vaccine trial
Pre-clinical study of new DNA vaccine showing 100% protection against herpes simplex virus 2
Major milestones due within the next six months
• US regulatory filing update for CardioCel®
• Follow up data for heart valve reconstructions
• Data in pelvic floor and hernia repair
• Initial animal data for HPV vaccine programs
• Initiation of DNA HSV vaccine Phase I study
• Initial revenues from CardioCel® (first product from the regenerative medicine franchise)
The company continues to see some good revenue streams through its medical product supplies business unit, with additional income also being anticipated for FY2013 when CardioCel receives CE marking to commence European sales, and additional TGA authorisations are also expected over the near term. It is quite acceptable when looking at some of the significant milestones being acheived, as well as anticipated news flow due over the short term, for one to believe that AHZ share holders may soon be getting spoilt this Christmas, and most certainly early in the new year.
In my personal view, Prof Frazer's highly anticipated DNA Vaccine Trial due to commence in the short term, will attract substantial amount of media attention for a number of reasons: Prof. Frazer is already one of Australia's most successful and highly respected scientists, Australian of the year in 2006 and this year he received the highest accolade in the 2012 Queen's Birthday honours in June when he was appointed a Companion of the Order of Australia. Governor-General Quentin Bryce
said Professor Frazer's vaccine had changed the world.
Prof. Frazer, who invented the world's first cancer vaccine is now showing the same confidence in the development of his pioneering DNA vaccine against herpes simplex virus 2 (HSV-2) with results from a pre-clinical efficacy study already showing it to be 100% effective. Of course there are no guarantees in life, however there is also no alternative drug currently available to the millions of HSV-2 sufferers around the world today. One only needs to read the many comments posted in this article
to appreciate that millions of people will be hoping and praying for a successful outcome to these clinical trials, soon to get underway - I wish Prof. Frazer and his great team of research scientists all the very best of luck. Here's a qualitative analysis for Allied Health Group (ASX:AHZ) which currently hold 44% interest in Prof. Frazer's company, Coridon Pty Ltd
Allied Healthcare Group has three key strategic business drivers that support its vision.
Investing in next generation technologies with global potential, developed by world-class experts. We are committed to funding unique research programs by global leaders such as Prof. Ian Frazer.
Acquiring strategic and synergistic assets to grow our product and service offerings. We are continually assessing new opportunities to add to the Allied Healthcare Group – assets that will enhance our pipeline and add to shareholder value.
Expanding our revenue from our existing medical sales, marketing and distribution business. The growing healthcare market provides a strong platform for continued expansion into new and existing market segments as well as the opportunity to develop new products and services.
|
|
Pioneering DNA vaccine technology for the prevention of infectious diseases and cancers
View More... |
|
| Clinical development of regenerative tissue engineering technologies View More... |
|
| Sales and distribution of niche medical technologies View More... |
Allied Medical Ltd was originally spun out of Fortescue Metals Group Limited (FMG) and became an independent entity in November 2005.
Allied’s growth strategy has been founded upon leveraging its core competencies in sales, distribution and commercialisation to support its investments in growth areas –sectors identified as having the potential to make a significant and positive contribution in the healthcare sector.
In 2008, Allied identified a partnership to gain entry in the growing vaccine market with a right to acquire a majority shareholding in Coridon Pty Ltd (Coridon), a company founded by Professor Ian Frazer, Australian of the Year 2006. Professor Frazer is well recognised for his ground breaking research that led to Merck & Co.’s highly successful cervical cancer vaccine, Gardasil®.
The Coridon partnership fitted well with Allied’s long term strategic goal of creating shareholder value through identifying opportunities and investing in innovative technologies that have global potential with world class research expertise.
In 2010, the Allied expanded its sales and distribution business with the acquisition of Medevco Pty Ltd. Medevco is an Australian-based supplier and distributor of medical devices and has a sales, marketing, logistics and technical service footprint across Australia and New Zealand.
Product Portfolio & Research Pipeline
The medical sales, marketing and distribution business
Allied Medical is an Australian owned supplier and distributor of quality, innovative medical devices and best of class products and services to the Australasian Healthcare market.
An Australian based supplier of high quality medical devices and equipment with a sales, marketing, logistics and technical service footprint across Australia. Our dedicated sales team of industry specialists are supported in the field by a network of clinical educators who enhance the relationship with our customers. Our innovative, cost effective high quality niche devices have broad scope across many high profile medical specialties. With an established but growing revenue stream, the business is being positioned for expansion and will become a major force in the Australian Healthcare landscape. Currently expanding into New Zealand, Allied Medical is poised for growth across Australasia.
Allied Medical believes that the market is ready for a dedicated cardiology player who can perform to higher levels of service than the large corporations.
By combining the strengths of major manufacturers, Allied Medical is positioned to appeal to Australasian Medical Practitioners by offering leading edge, premium cardiology products which add value to their business and offer improved patient outcomes.
Cardiac Science 
Cardiac Science's sole focus is diagnosing and treating cardiovascular disease. This company is proud to position themselves as the monitoring company that builds cardiology products with the cardiologist in mind. Cardiac Science markets under these well-respected brands:BurdickFor more than 45 years Burdick has been a leading brand of ECG equipment in the primary care market and hospital market.
PowerheartPowerheart brand AEDs provide reliable, safe, and intelligent cardiac monitoring and defibrillation technology that significantly reduces defibrillation time to save lives.
QuintonQuinton is one of the most respected and recognised cardiac brands and is a leader in both cardiac stress testing and cardiac rehabilitation management systems for acute care and outpatient clinics.
SunTech 
SunTech is at the forefront of non-invasive blood pressure innovation technology that provides the highest possible level of accuracy and reliability. Their blood pressure boards and algorithms are found in almost every vital signs monitor in the world. Key clinicians recognise the SunTech product as the benchmark for Ambulatory Blood Pressure Monitoring (ABPM) and patent monitoring systems.Quinton Q-Stress
 Cardiac Stress System Quinton Q-Stress offers extensive customisation to meet the needs of all types of users. Depending on your facility, practice, procedure and reporting needs, Q-Stress can... Read more
Cardiac Stress System Quinton Q-Stress offers extensive customisation to meet the needs of all types of users. Depending on your facility, practice, procedure and reporting needs, Q-Stress can... Read moreQuinton TM 55/65 Treadmills
 Integrating with Quest, Q-Stress and Tango systems, the TM55 and TM65 treadmills deliver the accuracy and reliability required for exercise stress testing. Fewer moving parts reduces wear and... Read more
Integrating with Quest, Q-Stress and Tango systems, the TM55 and TM65 treadmills deliver the accuracy and reliability required for exercise stress testing. Fewer moving parts reduces wear and... Read moreSunTech Cycle
 Specifically designed for use in bicycle ergometer cardiac stress testing, the Cycle TM Monitor reliably monitors BP allowing you to focus on your patients. The new Cycle TM stress BP monitor is... Read more
Specifically designed for use in bicycle ergometer cardiac stress testing, the Cycle TM Monitor reliably monitors BP allowing you to focus on your patients. The new Cycle TM stress BP monitor is... Read moreSunTech Tango
 Specifically designed for use in treadmill, ergometer and pharmacological stress testing, the Tango reliably monitors blood pressure where standard monitors simply do not work. Interfaced with... Read more
Specifically designed for use in treadmill, ergometer and pharmacological stress testing, the Tango reliably monitors blood pressure where standard monitors simply do not work. Interfaced with... Read moreBurdick 8500 ECG
Quinton MedTrack CR60 Rehab Treadmill
 Designed specifically for rehabilitation facilities, this treadmill features an exclusive TripleFlex© shock absorbing deck that provides ideal cushioning for walkers and runners of all... Read more
Designed specifically for rehabilitation facilities, this treadmill features an exclusive TripleFlex© shock absorbing deck that provides ideal cushioning for walkers and runners of all... Read moreQuinton Q-Tel Rehabilitation Management Software
 The Q-Tel RMS system delivers comprehensive cardiac monitoring and data management. Designed with efficient workflow in mind, the Windows-based software provides familiar icons and an intuitive... Read more
The Q-Tel RMS system delivers comprehensive cardiac monitoring and data management. Designed with efficient workflow in mind, the Windows-based software provides familiar icons and an intuitive... Read moreSuntech 247 Diagnostic Station
 The SunTech 247 is a blood pressure monitoring system that provides both an affordable replacement for mechanical gauges and mercury sphygmomanometers with the potential of a vital signs device... Read more
The SunTech 247 is a blood pressure monitoring system that provides both an affordable replacement for mechanical gauges and mercury sphygmomanometers with the potential of a vital signs device... Read moreSunTech Oscar 2
24-Hour Ambulatory Blood Pressure Monitoring System The Oscar 2 Ambulatory Blood Pressure (ABP) monitoring system has been designed with both the clinician and the patient in mind. It... Read more
In the critical moments of a cardiac emergency you need to be able to rely on a superior product with a proven track record and unique features that result in better patient outcomes.Choose a Powerheart Defibrillator configured to suit your situation.
To download a product brochure, click HERE.
To download a product brochure, click HERE.
Volumed uVP 7000 Premium
 Premium Volumetric Infusion pump for general and highly specialised wards with features such as: Drug-dose-calculation Dose-error-reduction system Ramping Function Chart of drug dosage... Read more
Premium Volumetric Infusion pump for general and highly specialised wards with features such as: Drug-dose-calculation Dose-error-reduction system Ramping Function Chart of drug dosage... Read more
arcomed ag - switzerland :: Corporate Movie
UniQueCONCEPT - Infusion Data Management System
 UniQueCONCEPT™ the revolutionary Infusion Data Management System (IDMS) increases patient safety, simplifies workflow processes and enhances hospital’s legal certainty. By using... Read more
UniQueCONCEPT™ the revolutionary Infusion Data Management System (IDMS) increases patient safety, simplifies workflow processes and enhances hospital’s legal certainty. By using... Read moreUniQueDOC Docking Station
 The modular design of the docking stations allows you to stack pumps in one place and save space. Placing and removing the pumps is quite simple. The integrated mains power supply... Read more
The modular design of the docking stations allows you to stack pumps in one place and save space. Placing and removing the pumps is quite simple. The integrated mains power supply... Read moreDriving New Frontiers in Reconstructive And Regenerative Tissue Surgery
- Repair of Congenital Heart Defects
- Reconstructive Heart Valve Repair
- Abdominal Hernia Repair and Pelvic Floor Reconstruction
This unique technology enables Celxcel to be positioned as a world leading producer of treated tissue and regenerative products for repair of congenital heart defects, defective heart valve leaflets and ultimately the valves themselves. Our technology also has applications in general, orthopaedic and neuro surgery.
Allied Healthcare announces positive 2 & 3 year Phase II follow up data from CardioCel® Clinical Trial
* Patients showed no significant calcification over 3 years
* No other adverse issues with the CardioCel patches
In April 2012 Celxcel released positive 36-month post Phase II human clinical trial follow-up results of paediatric patients who received ADAPT® tissue engineered CardioCel® patches during various corrective cardiac surgical procedures.
CardioCel® is a collagenous-based bioprosthetic device, made of tissue derived from bovine pericardium and treated with Celxcel’s ADAPT® Tissue Engineering Process.
The follow up program followed selected patients to evaluate over a 3-year period. To date 14 patients have been followed up after 2 years and 5 patients have reached 3-year follow up points. All patients were free of patch-related complications or adverse events. Echocardiographic (heart ultrasound) results showed intact haemodynamics (blood flow) with no evidence of significant calcification of the CardioCel® patch at the 24 and 36-month evaluation.
"The follow up data further supports the data for the Celxcel technology, in particular the prevention of calcium build up in tissue post implantation," stated Allied Healthcare Group MD Lee Rodne. "This is a major market differentiator for products using the technology and provides major benefit for patients longer term."
In May, 2008, 30 patients (Age range; 3months - 14 years) were implanted with the ADAPT® engineered CardioCel® patches. The study included paediatric patients diagnosed with congenital heart disease that required a tissue patch for corrective surgical repairs during major open-heart surgery
The follow-up and evaluation of these patients has been under the supervision of the Principal Investigator and the paediatric cardiology team at the Universitas Hospital, Bloemfontein, South Africa. The follow up patients were provided a cardiac clinical evaluation, a full blood count, and an echocardiographic (Heart ultrasound) study to evaluate haemodynamic compatibility, evidence of calcification and the general efficacy of the CardioCel ® patch.
"Following these encouraging results we will be actively reviewing all patients in the Phase II study in order to get valuable additional long-term data for these paediatric CHD patients," said Bob Atwill CEO of Celxcel.
Congenital Heart Disease
Congenital Heart Disease is the most common type of birth defect, affecting approximately 8 in 1000 births. The number of older children and adults with some form of congenital heart defect are being identified more easily as technology improves.
Indeed the whole approach for treating increasing numbers of congenital heart defects is shifting as techniques improve, therapy becomes increasingly effective allowing for earlier interventions and more often, the treatment of older patients.
Calcification of the treated area is a common hurdle to overcome in the process as it reduces the life of the repair as the immune system can reject the implant. Celxcel's ADAPT® process reduces the calcification and extends the life of the repair as it closely mimics human tissue resulting in better performance and acceptance in the human body.
Celxcel has successfully completed a number of animal studies and a Phase II human clinical trial in the development of its lead product CardioCel®.
CardioCel® is a cardiovascular patch used to repair paediatric heart deformities. These deformities range from routine“Hole in the Heart” operations to major vessel outflow tract repairs. The CardioCel® patch may also be used to repair leaking heart valves in paediatric and adult patients.
Indeed the whole approach for treating increasing numbers of congenital heart defects is shifting as techniques improve, therapy becomes increasingly effective allowing for earlier interventions and more often, the treatment of older patients.
Calcification of the treated area is a common hurdle to overcome in the process as it reduces the life of the repair as the immune system can reject the implant. Celxcel's ADAPT® process reduces the calcification and extends the life of the repair as it closely mimics human tissue resulting in better performance and acceptance in the human body.
Celxcel has successfully completed a number of animal studies and a Phase II human clinical trial in the development of its lead product CardioCel®.
CardioCel® is a cardiovascular patch used to repair paediatric heart deformities. These deformities range from routine“Hole in the Heart” operations to major vessel outflow tract repairs. The CardioCel® patch may also be used to repair leaking heart valves in paediatric and adult patients.
Pelvic Floor Reconstruction
Synthetic meshes have been increasingly used in pelvic floor repair but as they do not necessarily behave like normal tissue, outcomes have often been controversial as they can cause a range of complications that include pain and even erosion of surrounding tissue. In addition immune responses to synthetic meshes can lead to problems of adhesion which often require further surgical intervention or repair.
Celxcel is working with a leading team of Gynaecologists from the University of Leuven in Belgium to trial Celxcel’s biocompatible tissue matrix GyneCel in animal implant models.The implants are currently being monitored for 6 months to look at the benefits of the GyneCel® patch compared to existing synthetic implants with follow up data to be released later in 2012.
Celxcel is working with a leading team of Gynaecologists from the University of Leuven in Belgium to trial Celxcel’s biocompatible tissue matrix GyneCel in animal implant models.The implants are currently being monitored for 6 months to look at the benefits of the GyneCel® patch compared to existing synthetic implants with follow up data to be released later in 2012.
Abdominal Repair
Ventral hernia repair is the most common form of abdominal repair. These procedures currently utilise both synthetic and biological meshes. The use of xenograft meshes have been well adopted and this together with the growth in elderly populations and the rise in obesity are expected to drive rapid growth in demand.
Celxcel’s ADAPT® technology has a significant role to play in the development and production of a more compliant and functional abdominal patch that has none of the deficiencies of the products currently used.
In turn, patients can have more confidence that post operatively they will be able to see greater improvement in their quality of life.
Celxcel’s ADAPT® technology has a significant role to play in the development and production of a more compliant and functional abdominal patch that has none of the deficiencies of the products currently used.
In turn, patients can have more confidence that post operatively they will be able to see greater improvement in their quality of life.
Cardiovascular Repair
In the US alone more than 1.4 million people are diagnosed with some form of heart valve disorder every year. Age and lifestyle factors that impact on the health of the population, combined with continually improving surgical techniques make it possible for greater numbers of patients, over a greater spread of age groups to be treated routinely.
Celxcel’s heart valve replacement tissue, CardioCel®,has the potential to greatly increase the lifespan of implanted bio-prosthetic tissue, either as part of a valvular reconstruction or ultimately to replace the faulty valve itself.
In the United Kingdom tissue valves are the preferred valve choice in the repair of aortic valves, with 70% of procedures being undertaken with bio-prosthetic tissue, despite the probable need for future replacement being outweighed by removing the need for long-term anti-coagulation therapy associated with mechanical valves.
The efficacy of CardioCel® as a heart valve replacement tissue is currently being tested in an animal model. Animal studies to assess the potential application of the CardioCel® device in the reconstruction of damaged or congenitally deformed heart valves are being undertaken by paediatric cardiothoracic surgeons at the University of Melbourne. The study aims to demonstrate the superiority of the CardioCel® heart patch as a valvular substitute in both the low pressured pulmonary valve and high pressured mitral valve.
Celxcel’s heart valve replacement tissue, CardioCel®,has the potential to greatly increase the lifespan of implanted bio-prosthetic tissue, either as part of a valvular reconstruction or ultimately to replace the faulty valve itself.
In the United Kingdom tissue valves are the preferred valve choice in the repair of aortic valves, with 70% of procedures being undertaken with bio-prosthetic tissue, despite the probable need for future replacement being outweighed by removing the need for long-term anti-coagulation therapy associated with mechanical valves.
The efficacy of CardioCel® as a heart valve replacement tissue is currently being tested in an animal model. Animal studies to assess the potential application of the CardioCel® device in the reconstruction of damaged or congenitally deformed heart valves are being undertaken by paediatric cardiothoracic surgeons at the University of Melbourne. The study aims to demonstrate the superiority of the CardioCel® heart patch as a valvular substitute in both the low pressured pulmonary valve and high pressured mitral valve.
Stem Cell Delivery
Celxcel has developed bio-prosthetic scaffolds or extra-cellular matrices that are able to support damaged tissue whilst it repairs and re-models in it’s own time within the body. This is a particularly exciting opportunity for Celxcel as research is focused on the seeding and implantation of stem cells within these matrices in order to demonstrate that ADAPT®treated matrices may provide a method of developing a range ofsite specific tissue implants. Currently Celxcel has developed the ADAPT® l and ll technologies that can generate matrices with different pore sizes which could ultimately lend to product of bioimplants with unique properties that more closely suit individual reconstructions.
Tissue matrices have been reported to play important roles in the regulation of stem cell differentiation and control of their fate.
Matrices or indeed, end products that can control their differentiation represent an attractive entry point into this market sector for Celxcel.The ADAPT® processes offer advantages in reduced glutaraldehyde usage (resulting in reduced calcification and reduced toxicity whilst retaining flexibility), controlled crosslinking and ability to generate tissue constructs with different porosities. These constructs with different tissue engineered properties together with high biocompatibility and controlled degradation provides a matrix-cell delivery platform with significant potential for use in regenerative medicine and biotherapeutics.
Life Scientist - Celxcel receives special access approval from TGA
Tissue matrices have been reported to play important roles in the regulation of stem cell differentiation and control of their fate.
Matrices or indeed, end products that can control their differentiation represent an attractive entry point into this market sector for Celxcel.The ADAPT® processes offer advantages in reduced glutaraldehyde usage (resulting in reduced calcification and reduced toxicity whilst retaining flexibility), controlled crosslinking and ability to generate tissue constructs with different porosities. These constructs with different tissue engineered properties together with high biocompatibility and controlled degradation provides a matrix-cell delivery platform with significant potential for use in regenerative medicine and biotherapeutics.
Celxcel Management
Bob Atwill - Chief Executive OfficerLeading the management team of Celxcel is Bob Atwill who has over 30 years of experience in the pharmaceutical, biotechnology and healthcare sectors. Bob has been a CEO and senior corporate officer in US, UK and Australian listed organisations. Prior to his appointment, Bob was CEO of Liquitab Systems Ltd and BioGrid Australia Ltd. He has also consulted to ASX-listed regenerative medicine company Mesoblast. Previously he served as the CEO & MD of Clinical Cell Culture, an ASX Listed company (now Avita Medical), Sales & Marketing Director of the Corin Group Plc and European Managing Director of the Sun Healthcare Group Inc. Bob has a BSc (Hons) in biochemistry from the University of Bristol, an Executive MBA from Ashridge Management College and has completed UCLA/Yale President Development courses.
Professor Leon Neethling - Chief Scientific Officer
Professor Leon Neethling - Chief Scientific Officer
Professor Leon Neethling was the founder of the first human heart valve bank in South Africa in 1984 and became Assistant-Director of the Cardiothoracic Research program in 1986. In 1993, whilst he was at the Prince of Charles Hospital in Brisbane, he received a Fellowship from the American College of Angiology and was appointed Director of the Cardiothoracic Research Program (Heart Valve Research Laboratory). In 1996, he received a Research Fellowship from the Heart-Transplantation Unit at Royal Perth Hospital to assess the kangaroo aortic valve as a possible heart valve substitute. From 1998 to 2007 Leon was appointed Associate Professor in Cardiothoracic Surgery at the Medical School in Bloemfontein, Director of Cardiothoracic Surgical Research at Fremantle Heart Institute in Fremantle, Western Australia and visiting Professor to the Department of Cardiothoracic Surgery in Bloemfontein, South Africa. During his career, he has received several prestigious academic awards and as an invited guest speaker has presented several national and international presentations in the field of cardiovascular related topics.
Geoff Strange - Vice President, Clinical & Medical AffairsGeoff Strange brings to Celxcel not only hands on clinical experience, but also a wealth of management experience. He has a keen interest in CHD and has recently set up the first Australian and New Zealand Adult CHD Consortium, responsible for the first adult ANZ CHD and PAH patient registry. This network includes the majority of medical specialists focused on adult CHD across Australia and New Zealand. Geoff has been intimately involved in the establishment of centre’s specialising in PAH and PHT management in Australia. He is also the CEO of the Pulmonary Hypertension Society of Australia and New Zealand. Geoff is currently a member of the Department of Cardiology research team at Royal Prince Alfred Hospital in Sydney, Australia, working directly for Professor David Celermajer. From 2002 – 2009 Geoff led the medical affairs team for Actelion Pharmaceuticals across the Asia Pacific region. He is currently the Managing Director of Mozaic Solutions Pty Ltd, a company set up to meet varying needs for medical professionals, pharmaceutical and device companies in clinical research, medical writing, medical marketing and key relationship management.
In addition to his peer reviewed publications, Geoff has co-authored the first ever pocket book ‘a physician’s guide’ to Pulmonary Arterial Hypertension management.
In addition to his peer reviewed publications, Geoff has co-authored the first ever pocket book ‘a physician’s guide’ to Pulmonary Arterial Hypertension management.
AHG’s CardioCel receives special access approval from TGA
The TGA has approved the first special access application for Allied Healthcare Group's (ASX:AHZ) CardioCel to help repair congenital heart defects in children.
- 26 September, 2012 16:40
Allied Healthcare has had a win in the step to see its congenital heart defect treatment, CardioCel, approved for use across Australia.
A Brisbane surgeon has had an application to use Allied Healthcare (ASX:AHZ) CardioCel patch in procedures approved under the Therapeutic Goods Administration's Authorised Prescriber Scheme.
This scheme enables medical practitioners to apply for use of a product before it has received formal marketing approval, once the product has been reviewed by their hospital.
This represents the first approval of the patch by the TGA for use within Australia.
Professor Tom Karl of the Mater Hospital in Brisbane – one of the first surgeons to submit an application to use CardioCel in his procedures – secured the inaugural approval to use it in procedures to repair congenital heart defects in children.
The authorisation gives a precedent for current and future applications by surgeons under the scheme.
It will also allow Allied Healthcare and its regenerative tissue engineering division, Celxcel, to start receiving profit from sales of CardioCel, albeit not necessarily the same percentage as when the product receives full marketing approval.
Allied Healthcare CEO of regenerative medicine Bob Atwill expects more approvals under the scheme in the coming months.
“A number of other key cardiothoracic surgeons are in [the] process of making similar applications to the TGA as a result of CardioCel’s preclinical and clinical data showing its effectiveness in treating congenital heart defects,” he said.
The CardioCel patch is produced using Celxel's ADAPT tissue engineering technology, which has the advantage of reducing the risk of calcification compared to other tissue-based products.
CardioCel also has applications in procedures including heart valve reconstruction.
Besides its TGA application, Allied Healthcare is seeking a CE Mark for Cardiocel in Europe and FDA approval in the US.
Life Scientist - Celxcel receives special access approval from TGA
A proven breakthrough in xenogeneic tissue engineering
Results from all pre-clinical animal studies indicate that the ADAPT® process works equally well on bovine, porcine and kangaroo derived tissues.
The key features and benefits of the ADAPT® technology include:
- Site‑specific controlled tissue remodelling – allows stem cell transdifferentiation1
- Conformation of the collagen triple helix – enables enhanced crosslinking within the collagen structure (masking of extracellular matrix (ECM) protein structure)
- Crosslinking with an ultra‑low monomeric polymer – provides ultimate strength and reduced inflammatory responses2
- Removal of all RNA and DNA including the α Gal Epitope –results in no antigen‑related immune responses.3
- Removal of all residual and unbound polymer molecules –provides complete biocompatibility.4,5,6
- Modification of all calcium binding sites – calcium levels reduced to physiological levels.
- Production of a stable soft tissue extracellular matrix (STEM)– providing a structural solution for graft durability and functionality.
The ADAPT® technology is a mature and patented platform technology that provides the following advantages from that of existing technologies:
- No foreign body response as with un‑crosslinked tissue grafts –results in increased biocompatibility.7
- No chronic inflammation as with un‑crosslinked tissue or synthetic grafts – results in increased biocompatibility and durability.8,9
- No rapid tissue degeneration as with un‑crosslinked tissue grafts – gives rise to functional graft maintenance.
Enhanced biostability and biocompatibility of decellularized bovine pericardium, crosslinked with an ultra-low concentration monomeric aldehyde and treated with ADAPT.
Source
Fremantle Heart Institute, School of Surgery and Pathology, University of Western Australia, Fremantle, Australia. Leon.Neethling@health.wa.gov.auAbstract
BACKGROUND AND AIM OF THE STUDY:
Matrix preparation remains controversial due to incomplete cell removal, inflammatory responses, reabsorption and thrombocyte activation. Previously, crosslinked matrices have been considered unsatisfactory due to cytotoxicity. In the present study, the biostability, biocompatibility and calcification potential of a decellularized matrix crosslinked with a low concentration of monomeric glutaraldehyde (GA) and treated with the ADAPT anti-calcification process were examined.METHODS:
Bovine pericardium was decellularized with Triton X-100, deoxycholate, IgePal CA-630 and ribonuclease. The resulting matrices were allocated to either group I (control, n = 5), crosslinked in 0.2% polymeric GA + ADAPT, or to group II (treatment, n = 5), crosslinked in 0.05% monomeric GA + ADAPT. The physical properties, enzymatic degradation, histology and immunohistochemical staining of the tissues were monitored. The matrices were also implanted in the jugular vein of juvenile sheep for 200 days.RESULTS:
Complete acellularity was achieved. Biostability was significantly (p <0.01) enhanced in group II, but inflammatory responses were limited in both groups. Host fibroblasts infiltrated the periphery in group I and the entire matrix in group II. The luminal surfaces were free from thrombotic depositions and covered with endothelial cells. Both groups tested positive for Factor VIII, smooth muscle alpha-actin and vimentin. Tissue extractable calcium levels were low (group I = 1.02 +/- 0.39, group II = 0.86 +/- 0.22 microg Ca/mg tissue).CONCLUSION:
Low-concentration GA-crosslinked matrices proved to be stable. The immunoreactivity of both groups was low, with host cell infiltration, migration and trans-differentiation being optimized in those grafts crosslinked with an ultra-low monomeric GA concentration. Calcification levels were close to zero in both groups. Enhanced crosslinking and effective anti-calcification produce a biomaterial with advanced in-vivo tissue-engineering properties.A multi-step approach in anti-calcification of glutaraldehyde-preserved bovine pericardium.
Source
Fremantle Heart Institute, Fremantle Hospital, School of Surgery and Pathology, University of Western Australia, Fremantle, Western Australia. Leon.Neethling@health.wa.gov.auAbstract
AIM:
Bioprosthetic cardiovascular substitutes, manufactured from glutaraldehyde-preserved bovine or porcine tissues, are prone to calcification after implantation. The aim of the study was to evaluate the ultrastructure, material stability and calcification behaviour of glutaraldehyde-preserved bovine pericardium, treated with a multi-step anti-calcification process which addresses each of the major causes of calcification and tissue degeneration.METHODS:
Bovine pericardium samples were divided into 2 groups. Group I (control) consisted of tissue fixed with 0.625% glutaraldehyde and Group II (study group) consisted of tissue fixed with 0.625% glutaraldehyde and exposed to a multi-step anti-calcification process. Ultrastructure was examined by scanning electron microscopy and material stability was assessed by mechanical testing, shrinkage temperature and enzymatic degradation. Calcification was assessed by histology (Von Kossa stain) and by atomic absorption spectrophotometry in the subcutaneous rat model.RESULTS:
Bovine pericardium in the study group revealed less visible changes in the ultrastructure of the collagen matrix, improved material stability (P<0.05) and significantly (P<0.001) reduced calcification compared to control tissues (4.5+/-1.2 versus 136.03+/-11.39 ug/mg tissue).CONCLUSIONS:
In conclusion, results demonstrate that the multi-step anticalcification process improved the material stability and reduced the calcification potential of bovine pericardial tissue. These improvements in the quality of the bovine pericardium should enhance the long-term durability of the tissue as a bioprosthetic substitute for cardiovascular application.Mitigation of Calcification and Cytotoxicity
Coridon, a company focused on developing next generation DNA vaccines, was founded by Professor Ian Frazer, Australian of the Year 2006. Prof Frazer is well recognised for his groundbreaking research that led to Merck & Co.’s highly successful cervical cancer vaccine, Gardasil®.
Through Coridon, Prof Frazer is working to expand his success with Gardasil by developing vaccines from patented technology that can be applied to a range of infectious diseases in humans.
Coridon’s technology has the potential to lead to the development of vaccines that aid in both the prevention of initial infection (traditional vaccine); and the treatment of those already infected (therapeutic vaccine).Prof Frazer’s team already has a proven track record in pioneering cancer vaccines and the work now being undertaken is expected to result in the development of DNA vaccines that have the ability to improve the quality of life of patients across the world.
Coridon was established in 2000 to undertake the commercialisation of outcomes from Prof Frazer’s work. Allied Medical acquired the rights to a majority shareholding in Coridon in 2008.
Herpes Simplex Virus Type 2 (HSV-2)
HSV-2 is the major causative agent of genital herpes. This disease does not just cause discomfort to infected individuals but can have serious health implications for babies born to infected women and is believed to aid the transmission of human immunodeficiency virus (HIV). Current HSV treatment involves the use of antiviral drugs which can reduce, but not eliminate, outbreaks and shedding and therefore does not prevent spread of the disease. Coridon is currently in the pre-clinical phase of evaluating a combined prophylactic and therapeutic HSV-2 vaccine with a view to commencing a clinical studies in 2012.
Epstein Barr Virus (EBV)
EBV is the causative agent of infectious mononucleosis (IM; glandular fever) in young adults and has been linked with Burkitt’s lymphoma, nasopharyngeal carcinoma (NPC), Hodgkin’s disease, non-Hodgkin’s lymphoma and lymphoproliferative diseases in the immunosupressed. Coridon is undertaking research and development of a prophylactic/therapeutic vaccine for EBV: prophylactic for IM and post-transplant lymphoproliferative disease and therapeutic for recurrent infection as a cause of NPC (in Asia) and lymphoma (in Africa).
Human Papilloma Virus (HPV)
HPV is one of the most common sexually transmitted diseases in the world and, as well as cervical cancer, is associated with a variety of anogenital cancers and head and neck cancer. Cervical cancer is the second largest cause of cancer deaths in women worldwide. Coridon is undertaking research and development of a therapeutic vaccine to combat existing HPV infection and to prevent and treat cervical and other HPV-associated cancers. An effective therapeutic vaccine has enormous potential in both the treatment of patients and reduction in deaths from HPV-associated cancers.
Coridon’s optimisation technology could potentially be applied to the development of DNA vaccines for a range of infectious diseases and cancers. Furthermore, as the technology should stimulate not only a strong antibody response but also a robust cellular immune response, it is particularly suited to the development of therapeutic vaccines.
Coridon’s patented technology involves the use of:
Unique codon modification
Codons are the triplet nucleotide sequences in DNA and RNA which code for individual amino acids. The genetic code is degenerate in that the majority of amino acids are encoded by more than one codon e.g. there are four codons (GCU, GCA, GCC and GCG) for the amino acid alanine.
It is recognised that modifying the codon composition of a gene can affect the amount of protein produced from its mRNA. Coridon has developed a unique approach to codon optimisation that takes into account the observation that codon preferences vary according to cell type and differentiation state (Zhao et al., 2005). In particular, Coridon has compiled a table, termed the Immune Coricode, for maximising the antibody response to intradermally-delivered DNA vaccines.
Ubiquitination
This refers to the engineering of an antigen gene to include an ubiquitin-encoding sequence. The ubiquitin targets the antigen to the proteasome for processing into peptide fragments. This leads to an enhanced cellular immune response.
Combination of the two technologies should allow production of DNA vaccines which elicit both a strong antibody response and a robust cellular response. Designed to stimulate both arms of the immune response, the technology is ideally suited not only for the development of prophylactic vaccines but also of therapeutic vaccines.
Coridon Management
Prof. Ian H Frazer FAA FRCP(Ed), FRCPA, Chair and Non-executive Director
Professor Ian Frazer is a clinician and pathologist, trained in immunology at Edinburgh University and the Walter and Eliza Hall institute in Melbourne. His accomplishments include a 15-year track record of world-class research in viral immunology and vaccine development. He has a portfolio of eight completed patents. He has collaborated over 13 years with CSL Ltd and Merck & Co to commercialize a cervical cancer prophylactic vaccine, and to develop therapeutic vaccines for HPV infection. He was the first to identify the HPV L1 protein as a vaccine candidate. In 1999 he won an Australian Biotechnology Award for successful collaborative research leading to product commercialization. In 2004 he was inducted into the prestigious Australian Academy of Sciences in recognition of the significant contribution he has made to biomedical science.
Lee Rodne BA MBA, Non-executive Director
Lee Rodne has over 15 years of leadership experience in technology, healthcare, medical devices, and mining & renewable energy sectors in North America, UK and Australia. He is a results-driven leader with expertise in business development, strategic management, M&A and commercialising new technology-based initiatives. Lee has been in Executive leadership roles in both Public and Private enterprises and is the current Managing Director of Allied Healthcare Group Ltd. Lee holds an MBA from the University St. Thomas, Minnesota and a B.A. degree in Business Management from St. John's University, Minnesota.
Julian Chick PhD, Non-executive Director
Julian Chick, COO Allied Healthcare Group Ltd., is an experienced corporate executive with 10 years experience in senior management with several roles as CEO (including Avexa Ltd), Head of Business Development at Amrad, plus running early and late stage R&D projects, particularly in the area of infectious diseases. In the past 6 years Julian has raised over $170M for R&D. In addition, he had five years experience as an investment adviser and financial consultant with Prudential-Bache Securities, BNP Paribas and Salomon Smith Barney. Julian also was the principal analyst with Foursight Associates reviewing healthcare and biotechnology investment opportunities for private equity investors and venture capitalists. He has a PhD in Muscle Physiology from La Trobe University.
Andrew Davis BSc (Hons) MBA, Non-executive Director
Andrew Davis serves as a UniQuest-appointed member of our Board of Directors. He is General Manager Technology Commercialization at UniQuest, the technology transfer company owned by The University of Queensland. Andrew was General Manager of a technology startup 1995 -99 and worked on new venture development at Telstra 1991-94. He is a director of a number of other high tech early stage companies.
Dean Moss BSc PhD, Non-executive Director
Dean Moss serves as General Manager Life Sciences at UniQuest and serves as a UniQuest-appointed member of the Board of Directors. He has held executive positions at York Medical Technologies Ltd I and Unitech UK specializing in distribution and sales of medical scientific and surgical products. He has significant international business development experience.
Stephen Denaro B Bus QUT; Grad Dip App Corporate Governance, Company Secretary
Stephen is a qualified chartered accountant with previous public company CFO and Secretary experience with biotech and software companies. He has ASX listing experience, and has strong relevant corporate equity and debt funding management skills. Stephen assists with financial and governance reporting to the Board.
Management:
Neil Finlayson BSc (Hons) BCom, Chief Executive OfficerNeil Finlayson has extensive experience in banking and finance, JVs, partnering and licensing, and in company formations. Neil was formerly Business Development Director of Bio Innovation SA in Adelaide. During his career, Neil has licensed technologies to national and international biotech and pharmaceutical companies and been instrumental in the formation of 17 companies. He has previously held positions as CEO of Vaccine Solutions Pty Ltd (co-owned by CSL Limited and the Queensland Institute of Medical Research), Executive Officer of the CRC for Vaccine Technology (a $42M joint venture) and began his career in banking and finance with Westpac Banking Corporation.
Research Team:
Julie Dutton BSc (Hons) PhD, Senior Scientist
Julie Dutton has a first class honours degree in biotechnology from the University of New South Wales and completed her PhD at the Institute for Molecular Bioscience, University of Queensland in 2002. After spending postdoctoral time at UQ and the Johannes Gutenberg University, Mainz, Germany, she was initially employed as a research scientist by Coridon in 2004. Julie is now responsible for the management of Coridon’s scientific program and has a specialist certificate in biomedical research management from the University of Melbourne.
Yvonne Woo BSc (Hons) PhD, Research Scientist
Yvonne Woo has a first class honours degree and a PhD in immunology from the University of Queensland which focussed on polynucleotide vaccine research. Apart from experimental work she also assists with project management and compliance with GLP.
Bo Li BSc (Hons) PhD, Research Scientist
Bo Li joined Coridon in 2008 with a PhD in molecular immunology from the Agriculture and Life Sciences Division of Lincoln University, New Zealand.
Yan Xu MD, Research Scientist
Originally trained as a pathologist in China, Yan Xu is one of Coridon’s first employees and has extensive experience in molecular biology techniques, cell culture and immunoassays.
Queen's Birthday Honours List
Business in Brisbane
Brisbane Convention & Exibition Centre
Statement against Gene Patents
ACRF Interview
Australian of the Year 2006
Published Work:
Paradigm shifting vaccines: prophylactic vaccines against latent varicella-zoster virus infection and against HPV-associated cancer
Ian H Frazer, Myron J Levin
Abstract
We compare the design, mechanism of action, and clinical efficacy of two recently licensed paradigm shifting vaccines. Zostavax is the first vaccine licensed to prevent disease in patients already infected with a pathogen, and is contrasted with Gardasil and Cervarix, the first vaccines designed and licensed specifically to prevent cancers.
Human papillomavirus (HPV) vaccines (click Table 1 )
Pathophysiology and epidemiology of HPV infection
Papillomaviruses are a family of largely species-specific dsDNA viruses that productively infect keratinocytes. Infection is non-lytic. Most HPV genotypes replicate without producing evident host pathology or inducing a measurable immune response. The majority of humans are chronically infected with multiple genotypes of these “non-pathogenic” HPVs [1]. Some HPV genotypes, including HPV 1 and 2, induce hyper-proliferative lesions of keratinizing skin, commonly known as warts. These infections are almost universal in childhood. Other HPV genotypes, including HPV 6 and 11, induce warty lesions on mucosal surfaces, which are extremely common in young adults and are transmitted through sexual contact.
A limited subset of about 10 so-called “high risk” HPVs, transmitted through sexual contact, can initiate warty or non-warty epithelial dysplastic changes at mucosal surfaces, particularly in the genital tract [2]. Evidence of current or past infection with these HPV genotypes is found in the majority of sexually active individuals, though only 1–2% of infections become chronic and can then induce, over many years, invasive squamous or adenosquamous cancer of genital epithelia, particularly the cervix [3]. Nearly 100% of cervical cancers, and a substantial proportion of other anogenital squamous cancers, are associated with high risk HPV infections. HPV 16 and HPV 18 are the genotypes most commonly associated with anogenital cancer, accounting for about 70% of cervical squamous cancers [4]. HPV 18 is associated more commonly with adeno- and adenosquamous cervical cancer. Cancers associated with infection with high risk HPV are found at other sites, especially tonsillar crypt epithelium. Some HPVs of skin, notably HPV 5, 8, and 38 are associated with development of squamous cell skin cancer in patients with chronically impaired immune responses [5], and proliferative lesions induced by mucosal “low risk” HPV genotypes 6 and 11 are much larger and last longer in immunosuppressed patients [6]. These observations demonstrate that host immunity is necessary to control HPV infection.
Papillomaviruses encode two structural proteins, L1 and L2, and up to 8 non-structural proteins (Figure 1). Oncogenesis associated with high risk HPV infection occurs in association with high level expression of two non-structural proteins, E6 and E7, which together delay differentiation and promote proliferation of epithelial cells. E7 binds to and inhibits the function of pocket proteins including Rb, permitting cells to cycle and proliferate when no longer in contact with the epithelial basement membrane, while E6 enables replication of cells with damaged DNA by promoting ubiquitination and destruction of p53 [7]. Both proteins have other actions which destabilize host cell DNA. These proteins have similar actions in high and low risk HPV infections. Together, the E6 and E7 proteins of HPV 16, expressed as transgenes in keratinocytes are sufficient to induce epithelial tumours in mouse skin [8]. The reason why only some HPV infections persist, and why persisting high risk HPV infections can transform cells, remains uncertain. While immune suppression increases the risk of HPV persistence and malignant transformation of high risk HPV infections, the majority of patients with persistent HPV infection and cervical cancer have apparently normal innate, humoral, and cellular adaptive immune systems, though there is a suggestion that the adaptive cellular immune responses to HPV E2, E6 and/or E7 non-structural proteins may be impaired in patients with persisting HPV infection [9]. Malignant transformation is generally associated with integration of the HPV genome into cellular DNA and high level expression of E6 and E7 proteins. Little immune response to HPV nonstructural proteins is detected, except in invasive cancer [10]. Immune response to the major L1 capsid protein, which is the basis of current vaccines to protect against HPV infection, is weak and not always observed during or after natural infection [11, 12].

The natural history of papillomavirus infection in the cervix. Infection results in expression of viral non-structural proteins inducing minor alterations to epithelial architecture (CIN 1). Infectious virions in the nucleus of superficial epithelial keratinocytes are associated with nuclear abnormalities (koilocytosis). CIN 1 regresses over months to years in >95% of patients. However, for high risk HPV infections, integration of viral genetic material into cellular DNA can result in overexpression of the E6 and E7 nonstructural proteins, promoting genetic abnormalities. These increase over years to histologically evident nuclear atypia and failure of epithelial cell differentiation (CIN 3). Further genetic changes in keratinocyte DNA in CIN 3 lesions enables clones of cells to acquire invasive properties leading to cancer, in about 30% of patients. Cancer cells generally express only the E6 and E7 non-structural proteins of HPV (Figure adapted from [65]).
Steps leading to development of HPV vaccines
PVs are encapsidated in an icosahedral coat comprised of 360 copies of the L1 capsid protein, arranged as 72 pentamers, and a smaller but uncertain number of copies of the minor L2 protein [13]. Immune responses to the HPV capsid following natural infection are directed to conformational epitopes on the external surface of the virus [14, 15]. These responses are weak and are apparently not fully protective against further infection with the same virus genotype [16]. There are sequence differences in the L1 protein between HPV genotypes, which result in type-specific epitope presentation on the external face of the virus, such that genotype cross-reactive antibodies are seen only between closely related HPV genotypes.
The association of high risk HPVs with cervical cancer was first proposed by Prof Harald zur Hausen in the early 1980s [17] who found that HPV genetic material was integrated within 70% of cervical cancers. Infections with the high risk HPV genotypes was initially thought to be rare, though it has subsequently been established that high risk HPV infection is common after the onset of sexual activity, although persistence and progression of such infection to cancer is rare [18]. This, together with the association of a limited number of HPV genotypes with cervical cancer, led to interest in development of prophylactic vaccination. A major obstacle was the inability to replicate HPVs efficiently in vitro to produce a vaccine from live virus by heat or chemical inactivation. A non-live vaccine was considered preferable, since the association of HPV infection with malignancy raised concerns about the safety of any vaccine produced from a live attenuated virus. Fortunately, the then nascent technologies for expression of viral protein from recombinant DNA could be used to produce recombinant proteins from the HPV 16 genome. HPV L1 protein, when expressed in eukaryotic cells efficiently assembled into viral capsomers and empty capsids. To achieve this, the protein had to be expressed from the second potential translational start site in the L1 open reading frame [19, 20], and a L1 gene without a mutation present in the genome originally cloned from a cervical cancer was required [21]. Viral capsomer material produced in this manner, the basis of the currently available HPV vaccines, was demonstrated in early phase clinical trials to generate high titres of HPV-specific antibody in blood [22] and vaginal secretions [23], and to neutralize HPV virions in a number of in vitro assays. Antibody alone is sufficient for viral neutralization in vivo, as passive transfer of capsid-specific antibody is sufficient to protect dogs against challenge with canine oral PV [24]. Antibody-mediated viral neutralization in vivo may involve blockade of binding of PV capsids to receptors on cells or the epithelial basement membrane, and may also prevent furin-mediated proteolytic cleavage of the L2 capsid protein, necessary for infectivity [25]. A wide range of expression systems have been used, though yeast-based [26] and insect cell-based [21] systems are the most efficient and provide the basis of the current commercial vaccines. Addition of the L2 protein was initially thought desirable to enhance particle production [19] and to improve immunogenicity, but particle production with L1 alone is quite efficient in most systems, and L2 within capsids does not contribute measurably to the virus neutralizing antibody response to capsid-based vaccines [27].
Pivotal trials
Several phase II trials [28–30] of HPV virus like particle vaccines were conducted in the early 2000s, and demonstrated protection against high risk HPV infection. These were based on HPV 16 and HPV 18 empty capsids (virus like particles), adjuvanted with alum, with or without addition of toll-like receptor (TLR 4) agonist monophosphoryl lipid A. Subsequent pivotal phase III trials [31–34], which involved over 20,000 women, documented the ability of the vaccines to protect women naïve to high risk HPV 16 and HPV 18 from development of premalignant genital lesions induced by persisting virus in the cervix, vulva and anus, termed intraepithelial neoplasia (CIN, VIN, AIN) [35]. Efficacy data has been extended to show protection of older women naïve to HPV [36], and to show some cross protection against high risk genotypes not incorporated in the vaccines [37]. Remarkably, studies showed near 100% protection over as long as 8 years against infection and development of CIN [38]. Protection against new infection has also been demonstrated in women without current infection, but with serological evidence of past infection [16]. However, there has been no evidence of protection against development of CIN for the many women recruited to the pivotal trials with DNA evidence of anogenital HPV infection at the time of vaccination, even if no clinical disease was present at the time of immunization [39].
Immunogenicity as a surrogate for vaccine efficacy
High titres of neutralizing antibody to viral capsid were observed after three doses of vaccine in all women immunized in the pivotal trials. Antibody titres declined more than 90% over the first two years after vaccination, and then plateaued, but could be rapidly boosted by repeat immunization, demonstrating effective B cell memory responses [40]. After immunization against HPV 18, up to 50% of subjects had lost measurable antibody after 3 years, although vaccine efficacy was maintained during a 5 years follow-up period [41]. Thus, currently, no level or specificity of HPV antibody conveyed through natural infection or vaccination is validated as a surrogate measure of protection against subsequent virus challenge.
Indications and safety profile
Given the need to immunize prior to exposure to high-risk HPVs, and the worldwide global incidence of cervical cancer, with over 250, 000 annual deaths (Fig 2), the two licensed HPV vaccines are recommended for use in 12–14 year old girls globally. The vaccines are licensed in most countries, including developing countries, for females between the ages of 9 and 45 years. In many countries the recommendations include young males. Population-based programs for immunization have been introduced in the United States, Australia, Canada, the United Kingdom, many European countries, Bhutan, and Malaysia. Vaccine safety is excellent, with only local reactogenicity as a common side effect, and very rare allergic reactions reported (about 1 per 106 immunized subjects) [42, 43].
Global distribution of cervical cancer, and of HPV vaccine licensure. Upper Panel: Distribution of cervical cancer deaths world wide as reported through Globocan. Lower Panel: Licensure of the bivalent and quadrivalent vaccines by country in 2009.
Other issues and future considerations
One of the two commercially available vaccines also incorporates virus like particles of HPV 6 and 11, which together are responsible for 95% of genital warts. This vaccine was protective against genital wart disease in clinical trials [35]. Field efficacy in Australia, where between 2006 and 2008 approximately 80% of women between the ages of 12 and 26 received HPV vaccine, was demonstrated by a decline of more than 70% in the incidence of genital warts in young women, but not in men or in older women [44].
Some unanswered questions for the HPV vaccines are
- The duration of protection, which currently is under investigation [45], particularly in Finland and Australia, through linkage of cervical cytology and cancer registry data with a vaccination registry.
- The utility of immunizing both males and females to protect against other HPV-associated cancers, including genital, anal, and tonsillar cancers, for which protective efficacy could reasonably be predicted from the association of a high proportion of these cancers with HPV 16. Establishing efficacy through randomized clinical trials is unlikely because of the cost and size.
- The impact of mass vaccination on design of screening programs for early detection of premalignant cervical disease; this will become an important feature of cost-benefit analyses in some countries.
- Development of second generation vaccines incorporating up to seven more high risk HPV genotypes, which could give >95% protection against cervical cancer, and are currently in early phase clinical trial. It will be necessary to establish that the inclusion of multiple extra HPV genotypes will not impair induction of protection against the genotypes already incorporated in available vaccines [46]. Additional cost may also be an issue.
- The cost of implementing global immunization.
- The efficacy for prevention of reinfection and disease recurrence after surgical treatment of HPV-associated CIN, as the risk of malignancy arising from persistent HPV infection is partly genetically determined.
- The efficacy of HPV vaccines targeted at skin genotypes for prevention of skin cancer in sun exposed individuals.
- The possibility that two doses of vaccine might be sufficient to induce adequate duration of full protection; this is currently under trial.
- The efficacy of the HPV vaccine if given along with other pediatric vaccines, perhaps as a 2 dose regimen with a single adolescent booster [47]. Feasibility will be determined largely by proven duration of protection.
- Establishment of a surrogate (immunological) marker of current or future protection following vaccination.
- Establishment in humans of the efficacy of single protein vaccines against HPV-associated anogenital disease based on epitopes of the L2 capsid protein that are conserved among all high risk HPV genotypes and are protective in animals. These might provide a broad protection against cervical cancer at lower cost.
- Development of a therapeutic vaccine to prevent progression of persistent high risk HPV infection to cervical cancer.
Herpes zoster of right 5th cervical ganglion demonstrating dermatomal vesicular eruption
HZ occurs only in patients that previously developed varicella (chickenpox), usually during their childhood many decades earlier. The viremia that naturally occurs during varicella seeds the skin, causing the typical vesicular lesions on an erythematous base [53]. The virus that causes varicella and (ultimately) HZ, varicella-zoster virus [VZV]), is believed, based on clinical observations, to ascend in sensory axons that end at the base of varicella lesions [54]. By this route it reaches sensory neurons in cranial nerve and dorsal root ganglia. VZV may also reach these neurons as a consequence of the viremia. VZV remains latent in sensory neurons for the life of anyone that develops varicella. Approximately 5% of trigeminal ganglion neurons (and only neurons) contain latent VZV [55]. The VZV DNA copy number is usually 5–50 copies per neuron, indicating that VZV replication is blocked shortly after the neuron is infected.
Clinical evidence suggests that latent VZV can reactivate randomly in ganglia at unpredictable intervals [56]. Reactivation most often remains silent, but when the reactivating VZV is able to propagate in a ganglion it can cause a ganglionitis that involves many neurons and non-neuronal cells. This is the cause of the pain in the sensory distribution of the ganglion that precedes the skin eruption of HZ (termed “prodromal” pain). The replicating VZV can then descend in the sensory nerve to cause the characteristic dermatomal skin eruption of HZ. This in turn, is painful because of the progressive skin involvement - in addition to the ongoing ganglionitis. Even after the skin heals, the inflammation and disordered architecture of the involved ganglion can result in persistent pain (called postherpetic neuralgia) that can last for months. Although there is effective antiviral therapy for HZ, this natural history explains why antiviral therapy is almost invariably administered after considerable damage has been done in the sensory ganglion, especially considering the delay imposed by the prodromal stage and the unavoidable delay before the correct diagnosis is made and appropriate therapy administered.
The mechanism that normally maintains latency is not fully understood. However, it is clear that a robust immune response is required to maintain latency and/or to prevent propagation of reactivated VZV in ganglia. This is demonstrated by the high frequency of HZ in patients who are immune suppressed [57]. Clinical observations document that patients with an immune defect limited to antibody synthesis do not have an increase in incidence or severity of HZ, whereas those that lack T-cell-mediated immunity (eg, HIV, chemotherapy, or organ transplantation) have frequent and morbid HZ. This essential requirement for VZV-specific T-cell-mediated immunity also explains the relationship of age to the incidence of HZ, since VZV-specific T-cell-mediated immunity declines progressively with increasing age, starting about age 35 years, whereas VZV-specific antibody is well maintained into the 8th and 9th decade of life [58, 59].
Steps leading to developing HZ vaccine
It was hypothesized that the immune responses most likely to protect against HZ would be best induced by a live, attenuated VZV vaccine. From 1985 to 1994 a series of experiments demonstrated that such a live vaccine(Oka VZV strain) prepared to prevent varicella could be safely administered to elderly subjects and that this vaccine stimulated the desired type of immune response [60]. Dose-response experiments led to a formulation, ultimately chosen for development as the HZ vaccine, that was 14-times more potent (in terms of viral titre) than the varicella vaccine (MJ Levin et al., 99th Annual Scientific Assembly of the Southern Medical Association, San Antonio, TX, 2005). The HZ vaccine contains no adjuvant. Safety and immunogenicity studies were performed, including administration to elderly subjects with commonly encountered chronic diseases, such as diabetes or chronic obstructive pulmonary disease (K Schmader et al., American Geriatrics Society Annual Scientific Meeting, Chicago, IL, 2006). These early trials suggested that persistence of induced immune responses would be at least 5 years [48]. From 1994–1999 planning for the pivotal trial was undertaken, including validation of assessment tools for a pain endpoint [61]; for the VZV PCR used to establish the HZ diagnosis [62]; and protocol development.
Pivotal trial
The pivotal trial (1999–2004) was a double-blind, placebo-controlled trial involving 38,500 subjects at least 60 years of age (>40% were ≥70 years) [48]. Efficacy against HZ was 51%; efficacy against a composite measure of pain over time was 61%; and efficacy against postherpetic neuralgia (significant pain ≥90 days after onset of skin lesions) was 66.5% (Table 2). The vaccine was safe and persistence of efficacy was determined to be at least 7 years during a long-term follow-up study of a subset of the original cohort (K Schmader et al., In: Programs and Abstracts of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Annual Infectious Disease Society of America Meeting, Alexandria, VA, 2008). Cases of “breakthrough” HZ that occurred in vaccinees were caused only by latent wild-type VZV and not by vaccine-strain virus, indicating that vaccine strain VZV does not become latent in people that have pre-existing immunity (MN Oxman et al., Shingles Prevention Study Group, unpublished).
Therapeutic efficacy of the VZV therapeutic vaccine in the elderly
As with all vaccines for the elderly, the efficacy of the vaccine was less effective at preventing HZ in the older age strata (Table 2). However, the protection against HZ and postherpetic neuralgia remained considerable into the 9th decade. Moreover, the morbidity of HZ increases with age and people of advanced age are disproportionately more affected by HZ than younger patients. The vaccine attenuates the severity of HZ when it occurs in very old vaccinees, as demonstrated by measuring the effect of HZ on their activities of daily living (ADL; as a measure of quality of life). The HZ vaccine substantially preserved ADL in subjects over 80 years old who developed HZ [52].
The efficacy in subjects at least 60 years old was sufficient for many countries to license the HZ vaccine for individuals 50–59 years of age, arguing that the booster response would be even greater in younger vaccinees. In the United States licensure required an efficacy trial of 22, 400 subjects, which demonstrated an efficacy against HZ of 69.8% (K Schmader et al, submitted for publication).
Immunogenicity as a surrogate for vaccine efficacy
Unlike the HPV prophylactic vaccines, where the capsid-specific antibody response is believed to be the essential component for preventing infection by an exogenous pathogen, perhaps by transudation of antibody to potential sites of infection (sterilizing immunity), the T-cell-mediated immune response to the HZ vaccine is both necessary and sufficient for protection, and does so by maintaining the clinical latency of an endogenous pathogen. An immunology substudy of the pivotal trial documented the appearance and persistence of both effector and memory CD4 cells, as well as antibody, after vaccination [63]. As described for the HPV vaccine, immune responses peaked during the first year and then declined by about 50% before reaching a plateau at years 2 and 3 post-vaccination (Figure 5). The persistence of these responses correlated with the persistence of the clinical effect. The magnitude of the T-cell-mediated VZV response was a function of the age of the vaccinee, explaining the effect of age on the efficacy profile of the vaccine (Figure 6). A subsequent study of the immune response to HZ in placebo recipients who developed HZ confirmed the importance of the rapid appearance of VZV-specific T-cell-mediated immunity for preventing severe HZ and postherpetic neuralgia [64]. Although a correlation between protection against HZ and the level of the immune boost was demonstrated, no precise level was determined as a strong surrogate of protection.

Indications and safety profile
HZ is indicated in many developed countries for people ≥50 years of age. The addition of the 50–59 year target group is important because many people in this age group are employed and HZ is associated with significant absenteeism, and decreased function while at work (P Singhal et al., unpublished, [51]). Because this is a live, attenuated virus, potential vaccinees must be immune competent. This concern is not applicable to the HPV vaccine. Prior HZ is not a contraindication to vaccine administration, since it is argued that this may prevent second cases of HZ (which are uncommon, but not rare). Potential vaccinees should not have recently received blood products and must not be taking antiviral drugs active against VZV, since these would inhibit replication of the administered live vaccine virus.
Other issues and future considerations
The HZ vaccine is an important addition to the medical care of aging individuals. However, because of immune senescence, this vaccine, as is the case for other vaccines that protect elderly people (influenza; pneumococcal polysaccharide), is not fully protective. The limitations of the HZ vaccine increase with advancing age. This remains an obstacle to the complete prevention of HZ.
The major future questions are:
- The persistence of HZ vaccine efficacy remains uncertain (may well exceed 7 years, but this may be age-specific).
- Studies are underway in previously vaccinated elderly people to see if a booster dose of HZ vaccine might be valuable a decade after receiving a first dose.
- Additional formulations and delivery of HZ vaccine need to be studied to increase its immunogenicity, especially in people of advanced age; potential approaches are the addition of an adjuvant, use of a non-live recombinant vaccine, or intradermal administration of vaccine. The latter would also be of potential value for immunocompromised patients.
- HZ vaccine in most countries requires a freezer, even though a refrigerator-stable formulation exists. This formulation is lacking because making sufficient quantities of this live vaccine has been problematic, thus limiting supply in general, and preventing wide-spread availability of refrigerator-stable vaccine (which requires more VZV).
- HZ vaccine is expensive and public health systems in many countries have not been adapted to support widespread use of the vaccine. However, cost-benefit analyses for this vaccine in many developed countries support it use, especially in people between 60 and 80 years of age. Such analyses are underway for 50–59 year-old vaccinees.
- HZ vaccine could be important for mildly immune suppressed patients, and for those that will become immune suppressed. This needs to be determined by a series of targeted clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
1. de Koning MN, Struijk L, Bavinck JN, Kleter B, Ter SJ, Quint WG, Feltkamp MC. Betapapillomaviruses frequently persist in the skin of healthy individuals. Journal of General Virology. 2007;88(Pt 5):1489–1495.[PubMed]
2. Munoz N, Bosch FX, Castellsague X, Diaz M, de SS, Hammouda D, Shah KV, Meijer CJ. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. International Journal of Cancer. 2004;111(2):278–285.
3. Wright TC., Jr Natural history of HPV infections. J Fam Pract. 2009;58(9 Suppl HPV):S3–S7.
4. Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de SS, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16.[PubMed]
5. Pfister H. Chapter 8: Human Papillomavirus and Skin Cancer. Journal of the National Cancer Institute Monographs. 2003;2003(31):52–56.[PubMed]
6. Bouwes Bavinck JN, Berkhout RJ. HPV infections and immunosuppression. Clin Dermatol. 1997;15(3):427–437.[PubMed]
7. Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16(2):83–97.[PubMed]
8. Griep AE, Herber R, Jeon S, Lohse JK, Dubielzig RR, Lambert PF. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. Journal of Virology. 1993;67:1373–1384. [PMC free article][PubMed]
9. Van Poelgeest MI, Nijhuis ER, Kwappenberg KM, Hamming IE, Wouter DJ, Fleuren GJ, van der Zee AG, Melief CJ, Kenter GG, Nijman HW, et al. Distinct regulation and impact of type 1 T-cell immunity against HPV16 L1, E2 and E6 antigens during HPV16-induced cervical infection and neoplasia. Int J Cancer. 2006;118(3):675–683.[PubMed]
10. Jochmus-Kudielka I, Schneider A, Braun R, Kimmig R, Koldovsky U, Schneweis KE, Seedorf K, Gissmann L. Antibodies against the human papillomavirus type 16 early proteins in human sera: Correlation of anti-E7 reactivity with cervical cancer. Journal of the National Cancer Institute. 1989;81:1698–1704.[PubMed]
11. Carter JJ, Madeleine MM, Shera K, Schwartz SM, Cushing-Haugen KL, Wipf GC, Porter P, Daling JR, McDougall JK, Galloway DA. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Research. 2001;61(5):1934–1940.[PubMed]
12. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–1919.[PubMed]
13. Buck CB, Cheng N, Thompson CD, Lowy DR, Steven AC, Schiller JT, Trus BL. Arrangement of L2 within the papillomavirus capsid. Journal of Virology. 2008;82(11):5190–5197. [PMC free article][PubMed]
14. Orozco JJ, Carter JJ, Koutsky LA, Galloway DA. Humoral immune response recognizes a complex set of epitopes on human papillomavirus type 6 l1 capsomers. Journal of Virology. 2005;79(15):9503–9514. [PMC free article][PubMed]
15. Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus- like particles assembled from the L1 protein of human papillomavirus 16. Molecular Cell. 2000;5(3):557–567.[PubMed]
16. Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10)
17. zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–265.[PubMed]
18. Anttila A, Kotaniemi-Talonen L, Leinonen M, Hakama M, Laurila P, Tarkkanen J, Malila N, Nieminen P. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ. 2010;340:c1804. [PMC free article][PubMed]
19. Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185(1):251–257.[PubMed]
20. Frazer IH, Cox J. Finding a vaccine for human papillomavirus. Lancet. 2006;367(9528):2058–2059.[PubMed]
21. Kirnbauer R, Taub J, Greenstone H, Roden R, Dürst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. Journal of Virology. 1993;67:6929–6936. [PMC free article][PubMed]
22. Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, O'Brien D, Campbell M, White WI, Balsley J, et al. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. Journal of Infectious Diseases. 2001;183(10):1485–1493.[PubMed]
23. Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De GP. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. JNCI Journal of the National Cancer Institute. 2003;95(15):1128–1137.
24. Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proceedings of the National Academy of Sciences, USA. 1995;92(25):11553–11557.
25. Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynaecologic Oncology. 2010;118(1 Suppl):S12–S17.
26. Lowe RS, Brown DR, Bryan JT, Cook JC, George HA, Hofmann KJ, Hurni WM, Joyce JG, Lehman ED, Markus HZ, et al. Human papillomavirus type II (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. Journal of Infectious Diseases. 1997;176(5):1141–1145.[PubMed]
27. Roden RB, Yutzy WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–257.[PubMed]
28. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765.[PubMed]
29. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a Bivalent L1 Virus-Like Particle Vaccine in Prevention of Infection With Human Papillomavirus Types 16 and 18 in Young Women: A Randomized, Controlled Trial. Obstet Gynecol Surv. 2005;60(5):303–305.
30. Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278.[PubMed]
31. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170.[PubMed]
32. Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–1702.[PubMed]
33. Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–1868.[PubMed]
34. The Future 2 study group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New England Journal Of Medicine. 2007;356(19):1915–1927.[PubMed]
35. Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–339.[PubMed]
36. Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–1957.[PubMed]
*37. Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–935. This paper analyses the incidence of new cases of HPV infection not due to HPV types included in the vaccine in over 17000 vaccine and placebo recipients in a randomised trial over a period of several years and concludes that there wass a significant reduction in incident infections amongst the immunised women for some HPV types (particularly HPV31) recognised to be closely immunologically related to HPV 16 or HPV18. This is one of several studies showing cross-protection, which have however not been entirely consistent as to which HPV types are partially protected through vaccination. [PubMed]
38. Bonanni P, Boccalini S, Bechini A. Efficacy, duration of immunity and cross protection after HPV vaccination: a review of the evidence. Vaccine. 2009;27(Suppl 1):A46–A53.[PubMed]
39. Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Journal of the Americal Medical Association. 2007;298(7):743–753.
40. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25(26):4931–4939.[PubMed]
**41. Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26(52):6844–6851. The authors examine the correlation between levels of induced antibody to HPV amongst women immunised with the quadrivalent HPV vaccine and placebo recipients, and note that antibody declines with time, and, for HPV18, becomes unmeasurable in about 40% of subjects. However, despite the loss of antibody, there are no incident infections with HPV18 in the immunised group, whereas incident cases continue to occur over the same period in placebo recipients. [PubMed]
42. Markowitz LE, Hariri S, Unger ER, Saraiya M, Datta SD, Dunne EF. Post- licensure monitoring of HPV vaccine in the United States. Vaccine. 2010
*43. Brotherton JM, Gold MS, Kemp AS, McIntyre PB, Burgess MA, Campbell-Lloyd S. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ. 2008;179(6):525–533. The authors examined the nature of anaphylactic reactions reported to follow administration of the quadrivalent HPV vaccine as routine vaccination to young women in Australia and show a low incidence (<2 per 10^6) of true anaphylaxis, and a number of anaphylactoid reactions which were not reproduced on antigen rechallenge. A similar more recent study showed slightly lower incidence and confirmed that anaphylactoid reactions occur that are not reproducible on rechallenge. [PMC free article][PubMed]
**44. Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, Wand H, Fairley CK. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2010;11(1):39–44. The authors examine the trends in new presentations with genital warts at sexually transmitted disease clinics around Australia during the period before and after the introduction of the quadrivalent HPV vaccine as a routine adolescent vaccine and note reduction in presentations amongst the cohort of women likely to have been vaccinated, but not amongst other groups, suggesting field efficacy of the vaccine program. A more recent study from Australia currently in press in the Lancet Oncology has shown a small reduction in presentations with CIN amongst this cohort. [PubMed]
45. Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert PH, Reisinger K, Haupt RM. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD((R)) Vaccine. 2010;28(30):4719–30.[PubMed]
46. Zhang T, Xu Y, Qiao L, Wang Y, Wu X, Fan D, Peng Q, Xu X. Trivalent Human Papillomavirus (HPV) VLP vaccine covering HPV type 58 can elicit high level of humoral immunity but also induce immune interference among component types. Vaccine. 2010;28(19):3479–3487.[PubMed]
47. Vesikari T, Van DP, Lindblad N, Pfletschinger U, Radley D, Ryan D, Vuocolo S, Haupt RM, Guris D. An open-label, randomized, multicenter study of the safety, tolerability, and immunogenicity of quadrivalent human papillomavirus (types 6/11/16/18) vaccine given concomitantly with diphtheria, tetanus, pertussis, and poliomyelitis vaccine in healthy adolescents 11 to 17 years of age. Pediatr Infect Dis J. 2010;29(4):314–318.[PubMed]
**48. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. This describes the pivotal clinical trial that established the efficacy of the herpes zoster vaccine for preventing herpes zoster and postherpetic neuralgia. It also describes the safety of the vaccine and the persistence for at least 4–5 years of the protective effect. [PubMed]
49. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–9.[PubMed]
50. Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335(1):32–42.[PubMed]
51. White RR, Lenhart G, Singhal PK, Insinga RP, Itzler RF, Pellissier JM, Segraves AW. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics. 2009;27(9):781–92.[PubMed]
*52. Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WW, Zhang JH, Chan IS, Yeh SS, Levin MJ, Harbecke RM, et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc. 58(9):1634–41. The authors describe the attenuating effect of the herpes zoster vaccine (when the vaccine fails to prevent herpes zoster) by measuring the extent to which the vaccine preserves the activities of daily living in vaccinees that develop herpes zoster. [PMC free article][PubMed]
53. Satyaprakash AK, Tremaine AM, Stelter AA, Creed R, Ravanfar P, Mendoza N, Mehta SK, Rady PL, Pierson DL, Tyring SK. Viremia in acute herpes zoster. J Infect Dis. 2009;200(1):26–32.[PubMed]
*54. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 51(2):197–213. This is an excellent review of aspects of herpes zoster and the herpes zoster vaccine. It provides detail on the pathophysiology of herpes zoster and the information that establishes that T-cell mediated immunity is essential for preventing clinically apparent reactivation of latent zoster virus. [PubMed]
55. Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77(12):6979–87. [PMC free article][PubMed]
56. Quinlivan ML, Ayres K, Ran H, McElwaine S, Leedham-Green M, Scott FT, Johnson RW, Breuer J. Effect of viral load on the outcome of herpes zoster. J Clin Microbiol. 2007;45(12):3909–14. [PMC free article][PubMed]
57. Gnann JW. Herpes simplex and varicella-zoster virus infection after hematopoietic stem cell or solid organ transplantation. In: Bowden R, Ljungman P, Snydman D, editors. Transplant Infections. Lippincott, Williams & Wilkins; Philadelphia: 2010. pp. 391–410.
58. Takahashi M, Okada S, Miyagawa H, Amo K, Yoshikawa K, Asada H, Kamiya H, Torigoe S, Asano Y, Ozaki T, et al. Enhancement of immunity against VZV by giving live varicella vaccine to the elderly assessed by VZV skin test and IAHA, gpELISA antibody assay. Vaccine. 2003;21(25–26):3845–53.[PubMed]
*59. Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 201(7):1024–30. This article summarizes published information on the age-related decline in T-cell immunity in people with prior varicella infection, and provides additional confirmatory information from a large cross-sectional database. [PMC free article][PubMed]
60. Levin MJ, Hayward AR. The varicella vaccine. Prevention of herpes zoster. Infect Dis Clin North Am. 1996;10(3):657–75.[PubMed]
61. Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–56.[PubMed]
62. Harbecke R, Oxman MN, Arnold BA, Ip C, Johnson GR, Levin MJ, Gelb LD, Schmader KE, Straus SE, Wang H, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81(7):1310–22.[PubMed]
**63. Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197(6):825–35. This describes a substudy of the pivotal vaccine trial (reference 48) that defines the zoster vaccine-induced boost in specific immunity. It further describes the persistence of that boost for the duration of the trial and defines the magnitude of the boost as a function of the age of the vaccinee. [PubMed]
*64. Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–77. This article describes the zoster-specific responses after onset of an attack of herpes zoster. It establishes that the early appearance of zoster-specific T-cell-mediated immunity limits the severity of the herpes zoster, whereas the antibody response does not correlate with protection. Thus, zoster-specific T-cell-mediated immunity is necessary for control of latent zoster virus and recovery from reactivation of that virus. [PubMed]
65. Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annual Review of Immunology. 2011;29:111–38.
66. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748–53. [PMC free article][PubMed]
67. de Melker H, Berbers G, Hahne S, Rumke H, van den Hof S, de Wit A, Boot H. The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination. Vaccine. 2006;24(18):3946–52.[PubMed]
68. Hope-Simpson RE. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article][PubMed]
69. Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, Macintyre CR. Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine. 2009;27(4):520–9.[PubMed]
70. Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, Chen CC, Lee DD, Chang YT, Wang WJ, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol. 2009;89(6):612–6.[PubMed]
71. Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos L, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127(2):305–14. [PMC free article][PubMed]
72. Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20(19–20):2500–7.[PubMed]
Human papillomavirus 16 E7 protein inhibits interferon-γ-mediated enhancement of keratinocyte antigen processing and T-cell lysis.
Source
The University of Queensland Diamantina Institute, Princess Alexandra Hospital, Brisbane, Qld, Australia.Abstract
Infection of epithelium with human papillomavirus (HPV) 16 is generally prolonged, suggesting an ineffective virus-specific immune response, and prolonged infection promotes anogenital cancer. To determine whether poor antigen presentation by HPV-infected keratinocytes (KCs) contributes to prolonged HPV infection, KCs and KCs expressing HPV 16 E7 protein (E7-KCs) were compared for susceptibility to T-cell-mediated lysis directed to ovalbumin (OVA) processed for presentation by the KCs. Interferon (IFN)-γ efficiently enhanced susceptibility to lysis of KCs presenting OVA, but not of E7-KCs similarly presenting OVA. E7-KCs also exhibited impaired IFN-γ-induced upregulation of transcription of major histocompatibility complex class I antigen processing and presentation-associated genes, and of membrane SIINFEKL-H-2K(b) complexes. Thus, expression of HPV 16 E7 protein in KCs may inhibit enhancement by IFN-γ of KC sensitivity to T-cell lysis, by impairing antigen presentation.http://www.ncbi.nlm.nih.gov/pubmed/21232015
Regulation of immune responses to HPV infection and during HPV-directed immunotherapy.
Source
The University of Queensland Diamantina Institute, Princess Alexandra Hospital, Brisbane, Australia.Abstract
The recent development of vaccines prophylactic against human papillomavirus (HPV) infection has the potential to reduce the incidence of cervical cancer globally by up to 70% over the next 40 years, if universal immunization is adopted. As these prophylactic vaccines do not alter the natural history of established HPV infection, immunotherapies to treat persistent HPV infection and associated precancers would be of benefit to assist with cervical cancer control. Efforts to develop immuno-therapeutic vaccines have been hampered by the relative non-immunogenicity of HPV infection, by immunoregulatory processes in skin, and by subversion of immune response induction and immune effector functions by papillomavirus proteins. This review describes HPV-specific immune responses induced by viral proteins, their regulation by host and viral factors, and highlights some conclusions from our own recent research.NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins.
Source
University of Queensland Diamantina Institute, Princess Alexandra Hospital, Woolloongabba, Queensland 4102, Australia.Abstract
We recently demonstrated that CD1d-restricted NKT cells resident in skin can inhibit CD8 T cell-mediated graft rejection of human papillomavirus E7-expressing skin through an IFN-γ-dependent mechanism. In this study, we examined the role of systemically derived NKT cells in regulating the rejection of skin grafts expressing viral proteins. In lymph nodes draining transplanted skin, Ag-specific CD8 T cell proliferation, cytokine production, and cytotoxic activity were impaired by NKT cells. NKT cell suppression was mediated via CD11c(+) dendritic cells. Inhibition of CD8 T cell function did not require Foxp3(+) regulatory T cells or NKT cell-secreted IFN-γ, IL-10, or IL-17. Thus, following skin grafting or immunization with human papillomavirus-E7 oncoprotein, NKT cells reduce the capacity of draining lymph node-resident APCs to cross-present Ag to CD8 T cell precursors, as evidenced by impaired expansion and differentiation to Ag-specific CD8 T effector cells. Therefore, in the context of viral Ag challenge in the skin, systemic NKT cells limit the capacity for effective priming of adaptive immunity.γδ T cells augment rejection of skin grafts by enhancing cross-priming of CD8 T cells to skin-derived antigen.
Source
The University of Queensland Diamantina Institute, Princess Alexandra Hospital, Woolloongabba, Queensland, Australia.Abstract
Gamma delta T cells (γδ T cells) possess innate-like properties and are proposed to bridge the gap between innate and adaptive immunity. In this study, we explored the role of γδ T cells in cutaneous immunity using a skin transplantation model. Following engraftment of skin expressing cell-associated model antigen (Ag) (ovalbumin) in epithelial keratinocytes, skin-resident γδ T cells enhanced graft rejection. Although the effector function of CD8 T cells was intact in the absence of γδ T cells, cross-priming of CD8 T cell to graft-derived Ag was impaired in the absence of γδ T cells. The reduced graft rejection and graft priming of γδ T-cell-deficient mice was evident in both acutely inflamed and well-healed grafting models. Furthermore, expression of the CD40 activation marker on migrating dendritic cells was lower in TCRδ(-/-) mice compared with wild-type mice, regardless of the presence or absence of inflammation associated with grafting. These results indicate that γδ T cells enhance graft priming and consequently the likelihood of a successful immune outcome in the context of skin graft rejection, suggesting that γδ T cells may be an important component of immunity to epithelial cancers or infection.http://www.ncbi.nlm.nih.gov/pubmed/22358058
A randomized trial of immunotherapy for persistent genital warts.
Source
Princess Alexandra Sexual Health; Princess Alexandra Hospital; Brisbane, QLD Australia.Abstract
Aim: To determine whether immunotherapy with HPV6 L1 virus like particles (VLPs) without adjuvant (VLP immunotherapy) reduces recurrence of genital warts following destructive therapy. Trial Design A randomized placebo controlled blinded study of treatment of recurrent genital warts amenable to destructive therapy, conducted independently in Australia and China. Methods Patients received conventional destructive therapy of all evident warts together with intramuscular administration of 1µg, 5µg or 25µg of VLP immunotherapy, or of placebo immunotherapy (0.9% NaCl), as immunotherapy at week 0 and week 4. Primary outcome, assessed at week 8, was recurrence of visible warts. Results Of 33 protocol compliant Brisbane recipients of placebo immunotherapy, 11 were disease free at two months, and a further 9 demonstrated reduction of > 50% in total wart area. Wart area reduction following destructive treatment correlated with prior duration of disease. Among 102 protocol compliant Brisbane recipients of VLP immunotherapy, disease reduction was significantly greater than among the placebo immunotherapy (50% ± s.e.m. 7%) recipients for subjects receiving 5 µg or 25 µg of VLP immunotherapy/dose (71% ± s.e.m.7%) but not for those receiving 1 µg VLP immunotherapy/dose (42% ± 7%). Of 52 protocol compliant placebo immunotherapy recipients in Wenzhou, 37 were disease free at two months, and a further 8 had > 50% disease reduction. Prior disease duration was much shorter in Wenzhou subject (8.1 ± 1.1 mths) than in Brisbane subjects (53.7 ± 5.5 mths). No significant reduction in mean wart area was observed for the 168 Wenzhou protocol compliant subjects who also received VLP immunotherapy. Conclusions: This study confirms the findings in a previous open label trial that administration of VLP immunotherapy may assist in clearance of recurrent genital warts in patients for whom destructive therapy is unsuccessful and that unsuccessful destructive therapy is more common with increasing prior disease duration.Allied Healthcare announces Coridon license to vector technology
Coridon progressing towards Phase I study
Coridon licenses vector technology to support DNA vaccine program
Coridon contracts US facility to manufacture the Phase I vaccine
Brisbane, Australia, 17th April, 2012
Allied Healthcare Group (ASX: AHZ) announced today an update on the progress of Coridon, its investment company founded by Professor Ian Frazer and working on developing the next generation of vaccines. Included in these activities, Cordon has entered into a license agreement with Nature Technology Corporation (NTC) and has contracted VGXI Inc in the US for production of clinical material for the Phase I study scheduled to begin later this year.
NTC has specifically designed safe, minimalised and antibiotic-free selection vectors, which offer superior expression of recombinant proteins in mammalian cells. These vectors have been designed to comply with US Food and Drug Administration (FDA) and European Medicines Agency (EMA) regulatory guidance. The use of this vector improves the overall benefit of the vaccine by driving the in vivo transcription and translation of the genetic material.
"Access to this technology will allow the Coridon vaccine to fully maximise its gene expression and therefore improve the performance of the vaccine" stated Allied Healthcare CEO Mr Lee Rodne "these are important steps forward to the initial Phase I study for Coridon which will provide validation of the Coridon technology".
Coridon’s Herpes vaccine, which was recently announced to be 100% effective in protecting animals against Herpes Simplex Virus 2 infection, incorporates the NTC8485 antibiotic-free expression vector. The guidance from US and European regulatory bodies seeks to eliminate non-essential sequences and to avoid use of antibiotic resistance genes where feasible.
In addition to the vector technology, Coridon also receives access to use NTC’s HyperGRO fermentation technology, which provides for high yield and cost effective DNA production. The manufacturing of the Herpes vaccine utilising the HyperGRO technology has now commenced with VXGI Inc.
The Phase I study for Coridon’s herpes vaccine is scheduled to begin later this year.
Neil Finlayson, Coridon CEO said: "This is an important agreement for the company to be able to access this leading technology and cements the relationship we have built up with NTC dating back to late 2009."
Allied Healthcare announces Coridon license to vector technology
28 February 2012
Coridon to Develop HPV Therapeutic Vaccine
- Professor Ian Frazer leading Coridon program
- Allied Healthcare Group (AHZ) a major shareholder in Coridon
Brisbane, Australia, 28th February 2012: Coridon Pty Ltd announced today that it has embarked on the development of a next generation therapeutic Human Papillomavirus (HPV) vaccine. The program will be based on preliminary work by Coridon founder Professor Ian Frazer’s team and follows on from Professor Frazer’s work that resulted in the successful cervical cancer preventative vaccines - Gardasil®, marketed by Merck, and Cervarix, marketed by GlaxoSmithKline.
Coridon’s HPV vaccine has been designed to combat existing infection with the HPV virus, to prevent and treat cervical and other HPV-associated cancers, therefore improving on existing HPV vaccines by having a therapeutic advantage. Coridon will initially collaborate with the University of Queensland’s Diamantina Institute to test the vaccine in pre-clinical models that they have established.
Neil Finlayson, Coridon CEO said: "This collaboration is based on the use of our unique patented optimisation technology combined with Professor Frazer’s undoubted expertise and experience in vaccine development and Human Papillomavirus."
Coridon is developing the next generation of vaccines for the prevention and treatment for a range of infectious diseases and cancers in humans. Coridon’s DNA vaccine technologies differ from conventional vaccines in that they offer both preventative and therapeutic value and have the potential to be delivered with a range of adjuvants.
"The work by Professor Frazer’s team at Coridon has significant potential globally to treat those patients already infected with the virus, something the current vaccines cannot do," said Lee Rodne, MD of Allied Healthcare Group. "This is another example of the potential for Coridon’s technology in the prevention and treatment of a wide range of cancers and disease."
HPV is associated with several human cancers, most notably cervical cancer. Current HPV vaccines, such as Gardasil® have proven to be safe and highly effective; however they are not suitable for all people. Furthermore current HPV vaccines are not therapeutic, and there are a significant number of people already infected with HPV and at risk of developing HPV-associated cancers.
Coridon to Develop HPV Therapeutic Vaccine
11 October 2011
Coridon Demonstrates 100% Protection against Herpes Simplex Virus 2 using DNA vaccine
- Professor Ian Frazer leading Coridon program
- Allied Healthcare Group (AHZ) major shareholder in Coridon
Brisbane, Australia, 11 October 2011: DNA vaccine development company Coridon Pty Ltd. today announced that it had successfully completed pre-clinical efficacy testing of its prototype Herpes Simplex Virus 2 (HSV-2) vaccine, with outstanding results. The company will now look to progress the program into clinical studies.
Collaborating with Professor David Koelle and his colleagues at the University of Washington in Seattle, Coridon tested a number of different formulations of Coridon’s prototype vaccine. These proved 100% effective at protecting animals against HSV-2 infection, confirming an earlier study with the University of Washington which also demonstrated 90-100% protection against infection. These results were presented at the 5th Vaccine and ISV Annual Global Congress in Seattle last week.
Having achieved this milestone, Coridon has now secured additional funding from major investor Allied Healthcare Group and plans to commence manufacturing and undertake formal pre-clinical safety studies before testing the vaccine in a Phase I clinical study.
"The results of our herpes vaccine mark the beginning of an exciting period," stated Professor Ian Frazer, Chairman of Coridon. "Over the next 12 months, we expect pivotal data showing that our HSV vaccine, which incorporates Coridon optimisation technology, produces similar immune responses in the clinic to those seen in the animal trials."
Coridon is developing DNA vaccines for the prevention and treatment for a range of infectious diseases and cancers in humans, utilising the company’s patented technology. Coridon’s DNA vaccine technologies differ from conventional vaccines in that they offer both preventative and therapeutic value.
Coridon was founded by Professor Ian Frazer to commercialise his work in developing next generation DNA vaccines. Professor Frazer’s work at Coridon follows the success of his cervical cancer vaccine Gardasil. Major shareholder in Coridon, Allied Healthcare Group (ASX: AHZ) is working with Professor Frazer to assist in the commercialisation of his work.
Lee Rodne, MD of Allied Healthcare Group said: "These data provide fantastic validation to the Coridon platform which could be applied to a number of infectious diseases. We are excited about the path forward for the program as it moves toward clinical studies."
Coridon Demonstrates 100% Protection against Herpes Simplex Virus 2 using DNA vaccine
#2 Patents
Coridon / Prof. Ian H Frazer
|
NUCLEIC ACID SEQUENCE AND METHOD FOR SELECTIVELY EXPRESSING A PROTEIN IN A TARGET CELL OR TISSUE
A synthetic nucleic acid sequence and a method
are disclosed for selectively expressing a protein in a target cell or tissue of
a mammal. Selective expression is effected by replacing at least one existing
codon of a parent nucleic acid sequence encoding a protein of interest with a
synonymous codon to produce a synthetic nucleic acid sequence having altered
translational kinetics compared to the parent nucleic acid sequence. The
synonymous codon is selected such that it corresponds to an iso-tRNA which, when
compared to an iso-tRNA corresponding to the at least one existing codon, is in
higher abundance in the target cell or tissue relative to one or more other
cells or tissues of the mammal.
WO1999002694 - NUCLEIC ACID SEQUENCE AND METHOD FOR SELECTIVELY EXPRESSING A PROTEIN IN A TARGET CELL OR TISSUE
TREATMENT OF PAPILLOMAVIRUS INFECTIONS
This invention relates to treatment of
papillomavirus infections. Primarily there is provided a method of treatment of
an existing papillomavirus (PV) infection which includes the step of
administration of PV VLPs selected from the group consisting of PV L1 VLPs and
PV L1/L2 VLPs to a patient suffering from the PV infection. Suitably the PV
infection is characterised by the presence of epithelial lesions. The major
infection which is treated are genital warts caused by HPV 6 and HPV
11.
WO2000035478 - TREATMENT OF PAPILLOMAVIRUS INFECTIONS
METHOD AND POLYNUCLEOTIDES FOR DETERMINING TRANSLATIONAL EFFICIENCY OF A CODON
A method is disclosed for determining the
translational efficiency of an individual codon in a cell. The method comprises
introducing into the cell a synthetic construct comprising a reporter
polynucleotide fused in frame with a tandem repeat of said individual codon,
wherein said reporter polynucleotide encodes a reporter protein, and wherein
said synthetic construct is operably linked to a regulatory polynucleotide and
measuring expression of said reporter protein in said cell to determine the
translational efficiency of said codon.
WO2000042215 - METHOD AND POLYNUCLEOTIDES FOR DETERMINING TRANSLATIONAL EFFICIENCY OF A CODON
POLYNUCLEOTIDE AND METHOD FOR SELECTIVELY EXPRESSING A PROTEIN IN A TARGET CELL OR TISSUE OF A PLANT
A method is disclosed for constructing a
synthetic polynucleotide from which a protein is selectively expressible in a
target cell of a plant, relative to another cell of the plant. The method
comprises selecting a first codon of a parent polynucleotide for replacement
with a synonymous codon which has a higher translational efficiency in the
target cell than in said other cell, and replacing said first codon with said
synonymous codon to form said synthetic polynucleotide.
WO2000042190 - POLYNUCLEOTIDE AND METHOD FOR SELECTIVELY EXPRESSING A PROTEIN IN A TARGET CELL OR TISSUE OF A PLANT
Allied Healthcare Group signed a research collaboration with CSIRO focusing on the development of novel tissue engineering technologies which has the potential to aid tissue repair in the heart. The company has obtained support for the project from Enterprise Connect through the Researchers in Business to partially fund the research. The research will focus on the development of ADAPT® treated tissue matrices as scaffolds for the delivery of adult mesenchymal stem cells in models of heart failure.
To date studies indicate the ADAPT treated tissue offers advantages over other tissues in reduced calcification. The aim of this collaboration is to use the ADAPT technology to produce a new platform for the delivery of stem cells. Repair of cardiovascular tissue through provision of a tissue bioscaffold and the attraction of cells to repopulate and replace the initial scaffold would be anticipated to offer a superior, long lasting regenerative medicine implant that becomes native tissue. The technology could be applied across a range of medical conditions beyond cardiovascular applications.
"This development allows us to increase the undoubted commercial potential of the CardioCel® and ADAPT® technology and produce a new platform technology based on a combination of the Allied technology with stem cells. This builds further on the company strategy to bring multiple products to market for the multi-billion dollar regenerative medicine market" stated Lee Rodne Allied Healthcare Group Managing Director.
Allied’s regenerative medicine division has developed a novel tissue engineering technology called ADAPT® that provides acellular tissue matrices that have so far been utilised in the repair of congenital heart defects, valve replacement, hernia and pelvic floor repair. The tissues are fully compatible with the human body. The engineering process also provides the ability to regulate the porosity and associated properties of the tissue and this provides potential to design them for use as bioscaffolds for the delivery of stem cells.
Stem cells are recognised as important in tissue repair and regeneration and are believed to act through mechanisms including the recruitment of cells to the area for repair. Many studies demonstrate the matrix (properties and composition) on which the stem cells are seeded impact the type of new tissues formed.
CSIRO’s Biomedical Materials and Devices group has extensive experience in the development and evaluation of novel materials and surfaces for both the controlled expansion of stem cells and as scaffolds for stem cell matrices. The Researchers in Business grant enables Allied Healthcare Group and its subsidiary Celxcel to pursue a joint program with CSIRO Biomedical Materials and Devices group to assist with the development of its ADAPT® engineered tissues to generate the next generation of cardiac repair and regenerative products. This is the first of potentially many projects for Allied Healthcare Group in the regenerative medicine area.
Peter holds and Arts/Law degree from the Australian National University.
All information presented in this post has been sourced through publicly available information. In no way or form should my opinion be considered as investment advice. I am currently holding a position in AHZ and make no representation or warranty as to the accuracy of information posted on this blog, and encourage any potential investor of AHZ to follow up by conducting further research with any information which has been obtained from this site for the purpose of forming ones own investment decision - Best of luck with all your investments.
NOVEL COMPOSITIONS AND USES THEREFOR
The invention is directed to the use of (i) a
first antigen corresponding to a target antigen of interest, together with (ii)
a second antigen, corresponding to a modified form of the target antigen, whose
rate of intracellular proteolytic degradation is increased, enhanced or
otherwise elevated relative to the first antigen, in compositions and methods
for inducing both humoral and cellular immunity in an individual. The ability to
provide compositions, which are capable of inducing both host-protective
antibody and cell-mediated immune responses, facilitates the generation of
immunogenic compositions capable of combating, inter alia, conditions
that have long latency periods and, therefore, benefit from the dual approach of
prophylaxis and therapy in one delivery
GENE EXPRESSION SYSTEM BASED ON CODON TRANSLATION EFFICIENCY
The present invention discloses a method for
modulating the production of a protein from a polynucleotide in a CHO cell by
replacing at least one codon of the polynucleotide with a synonymous codon that
has a higher or lower translation efficiency in the CHO cell than the codon it
replaces, or by introducing into the CHO cell a polynucleotide that codes for an
iso-tRNA which limits the rate of production of the polypeptide and which
corresponds to a codon of the first polynucleotide. The present invention also
discloses the use of a protein-encoding polynucleotide whose codon composition
has been modified for enhanced production of the protein in CHO
cells.
A METHOD FOR OPTIMISING GENE EXPRESSION USING SYNONYMOUS CODON OPTIMISATION
The present invention discloses a method for
modulating the quality of a selected phenotype that is displayed by an organism
or part thereof and that results from the expression of a polypeptide-encoding
polynucleotide by replacing at least one codon of that polynucleotide with a
synonymous codon that has a higher or lower preference of usage by the organism
or part thereof to produce the selected phenotype than the codon it replaces.
The present invention is also directed to the use of a codon-modified
polynucleotide so constructed for modulating the quality of a selected phenotype
displayed by an organism or part thereof.
IMMUNOMODULATING COMPOSITIONS AND USES THEREFOR
The present invention discloses the use of an
inhibitor of IL-10 function and an immune stimulator that stimulates the priming
of an immune response to a target antigen, in methods and compositions for
stimulating and prolonging host immune responses to the target antigen. The
methods and compositions of the present invention are particularly useful in the
treatment or prophylaxis of a range of conditions including pathogenic
infections and cancers
CONSTRUCT SYSTEM AND USES THEREFOR
The present invention discloses construct
systems and methods for comparing different iso-accepting codons according to
their preference for translating RNA transcripts into proteins in cell or
tissues of interest or for producing a selected phenotype in an organism of
interest or part thereof. The codon preference comparisons thus obtained are
particularly useful for modifying the translational efficiency of
protein-encoding polynucleotides in cells or tissues of interest or for
modulating the quality of a selected phenotype conferred by a
phenotype-associated polypeptide upon an organism of interest or part
thereof.
EXPRESSION SYSTEM FOR MODULATING AN IMMUNE RESPONSE
The present invention discloses methods and
compositions for modulating the quality of an immune response to a target
antigen in a mammal, which response results from the expression of a
polynucleotide that encodes at least a portion of the target antigen, wherein
the quality is modulated by replacing at least one codon of the polynucleotide
with a synonymous codon that has a higher or lower preference of usage by the
mammal to confer the immune response than the codon it replaces
PAPILLOMA VIRUS VACCINE
A method of providing papilloma virus like
particles which may be used for diagnostic purposes or for incorporation in a
vaccine for use in relation to infections caused by papilloma virus. The method
includes an initial step of constructing one or more recombinant DNA molecules
which each encode papilloma virus L1 protein or a combination of papilloma virus
L1 protein and papilloma virus L2 protein followed by a further step of
transfecting a suitable host cell with one or more of the recombinant DNA
molecules so that virus like particles (VLPs) are produced within the cell after
expression of the L1 or combination of L1 and L2 proteins. The VLPs are also
claimed per se as well as vaccines incorporating the VLPs.
IMMUNOASSAY FOR CERVICAL CANCER
A method of stabilising HPVE7 protein in a
sample by boiling the sample and/or treating the sample with a denaturing agent
which may be sodium dodecyl sulphate which may also be employed in a final
concentration of between 0.01-0.1 %. The sample may be also boiled for 10-15
mins. prior to treatment by sodium dodecyl sulphate.
MODIFIED PAPILLOMA VIRUS L2 PROTEIN AND VLPs FORMED THEREFROM
The invention, in one aspect, is directed to a
modified papilloma virus L2 protein which does not bind DNA or binds a
substantially minimal amount of DNA. The invention is also directed to a method
of producing one or more virus-like particles which incorporates a substantially
minimal amount of DNA and the virus-like particles produced
therefrom.
RECOMBINANT PAPILLOMA VIRUS L1
This invention relates to a recombinant
papilloma virus L1 protein which can elicit an immune response which recognises
papilloma virus VLP including L1 protein and can form extracellularly a
multimeric structure or VLP wherein the multimeric structure comprises a
plurality of recombinant papilloma virus L1 proteins. This invention also
includes the use of the recombinant papilloma virus L1 protein to detect the
presence of papilloma virus and can form the basis of a vaccine for prophylactic
and therapeutic use.
NOVEL PROMISCUOUS T HELPER CELL EPITOPES
The invention is directed to a peptide
encoding a promiscuous T helper cell epitope for generating an immune response
against papillomavirus, wherein the peptide is selected from the group
consisting of (i) VYRDGNPYA inclusive of a single amino acid deletion,
substitution or addition made therein (SEQ ID NO. 1); and (ii) QYNKPLCDLL
inclusive of a single amino acid deletion, substitution or addition made therein
(SEQ ID NO. 2). The invention is also concerned with chimeric constructs as well
as immunogenic compositions comprising the peptides of the invention.
Celxcell / NEETHLING / HODGE
AN IMPLANTABLE BIOMATERIAL AND A METHOD OF PRODUCING SAME
The present invention relates to an
implantable biomaterial and methods of producing same. In particular, the
present invention relates to a method for producing an implantable biomaterial
comprising (a) exposing a biomaterial to an alcohol-containing solution for at
least 24 hours.
A METHOD USING POTASSIUM DIHYDROGEN PHOSPHATE TO REDUCE CALCIFICATION OF TISSUE
Potassium dihydrogen phosphate or a salt
thereof when used as a blocking agent and/or a buffer. More particularly, this
invention relates to a method of treating tissue to alleviate the occurrence of
calcification comprising the step of: (i) Contacting the tissue with a solution
of potassium dihydrogen phosphate or a salt thereof for sufficient time to allow
the potassium dihydrogen phosphate or salt thereof to impregnate the tissue,
wherein the potassium dihydrogen phosphate or salt thereof serves as a blocking
agent and/or a buffer.
Partnerships
Allied Healthcare Group & CSIRO
Stem cell collaboration for heart failure
Management
Chris Catlow - Chairman
Chris Catlow is an experienced executive in the capital markets. Over his 25 year career, he has held various senior roles in major operating companies and has considerable experience in raising both equity and debt for large projects. Chris was the inaugural CFO of Fortescue Metals Group Ltd and played a central role in its development and in raising more than US$4 billion. He was previously a director of Consolidated Rutile Ltd and Executive General Manager Finance of Iluka Resources Limited and is currently Chairman of Sirius Minerals Plc and Indo Mines Ltd. Chris has a Bachelor of Science in Engineering from the University of Durham, UK and is a Fellow of the Institute of Chartered Accounts in Australia.Lee Rodne - Managing Director
Lee Rodne has over 15 years of leadership experience in healthcare, technology, medical devices, and mining & renewable energy sectors in North America, UK and Australia. Lee has been in executive leadership roles in both public and private enterprises. He has served as a Director and Vice president of a U.S. based Healthcare Consulting & Distribution Company specializing on GE Healthcare products and services. Lee also led consulting services to the U.S. Healthcare, Device and Technology industries including Hospitals, Clinics, Multi-National Medical Device companies, Healthcare Insurance markets and various technology driven companies. Lee holds an MBA from the University of St. Thomas, Minnesota and a B.A. degree in Business Management from St. John’s University, Minnesota.Michael Bennett - Executive Director
Michael Bennett has over 35 years sales and marketing experience working for US and European medical device companies and has been involved in the introduction of many new medical and surgical device technologies to the Australian market. Michael worked with Ramsay Surgical Ltd, an Australian medical/surgical supply and distribution company until 1979. Since 1979, Michael owned and operated his own private surgical supply company, Bennett Medical, and exclusively represented several major overseas medical device manufacturing corporations. His company was involved in the introduction of many new surgical technologies to Australia. He sold the company in 2001. From 2001 he has been consulting to overseas surgical manufacturers and to the Australian medical industry in general.Graeme Rowley - Non-Executive Director
Graeme Rowley played a central role in the development of Fortescue Metals Group Ltd from its inception in 2003. He recently retired as an Executive Director of Fortescue but continues to serve as a Non-Executive of the company. Previously he was an executive with Rio Tinto Plc holding senior positions in Hamersley Iron and Argyle Diamonds. Graeme’s previous directorships have included Dampier Port Authority, the Pilbara Development Commission, the Council for the West Pilbara College of TAFE and the Western Australian State Government’s Technical Advisory Council.Peter Turvey - Non-Executive Director
Peter Turvey is currently a Principal of Foursight Associates Pty Ltd, a director of the industry organisation AusBiotech Ltd and a Non-Executive Director of Starpharma Holdings Ltd. Peter is the former Executive Vice President Licensing and Company Secretary of global specialty biopharmaceutical company CSL Ltd, having retired in 2011. He joined CSL in 1992 as its first in-house Corporate Counsel and was appointed Company Secretary in 1998. He played a key role in the transformation of CSL from a government owned enterprise, through ASX listing in 1994, to a global plasma and biopharmaceutical company. He also had responsibility for the protection and licensing of CSL's intellectual property and for risk management within CSL, reporting to the Audit & Risk Management Committee of the Board as well as being the Chairman of the Corporate Risk Management Committee. Peter was directly involved in the licensing cervical cancer vaccine, Gardasil to Merck & Co., the licensing of the Iscomatrix®adjuvant platform technology to the world’s leading vaccine manufacturers, and establishment of the P.gingivalis vaccine technology collaboration between the CRC for Oral Health and Sanofi-Pasteur.Peter holds and Arts/Law degree from the Australian National University.
Julian Chick - Chief Operating Officer
Julian Chick is an experienced corporate executive with 10 years’ experience in senior management with roles as Chief Executive Officer, Head of Business Development plus running early and late stage Research & Development projects. In the past six years Dr Chick has raised over $170 million for R&D projects. Julian had five years’ experience as an investment adviser and financial consultant with Prudential-Bache Securities, BNP Paribas and Salomon Smith Barney. He was also the principal analyst with Foursight Associates reviewing healthcare and biotechnology investment opportunities for private equity investors and venture capitalists. Julian has a PhD in Muscle Physiology.Bob Atwill - Group Executive and CEO Celxcel
Bob Atwill has over 30 years of experience in the pharmaceutical, biotechnology and healthcare sectors. Bob has been a CEO and senior corporate officer in US, UK and Australian listed organisations. Prior to his appointment, Bob was CEO of Liquitab Systems Ltd and BioGrid Australia Ltd. He has also been consulting to ASX-listed regenerative medicine company Mesoblast . Previously he served as the CEO & MD of Clinical Cell Culture, an ASX Listed company (now Avita Medical), Sales & Marketing Director of the Corin Group Plc and European Managing Director of the Sun Healthcare Group Inc. Bob has a BSc (Hons) in biochemistry from the University of Bristol, an Executive MBA from Ashridge Management College and has completed UCLA/Yale President Development courses.Stephen Mann - Chief Financial Officer and Company Secretary
Stephen Mann has more than seven years experience in commerce and public practice and has held senior finance positions with Bus and Coach International Pty Ltd and JV Global Ltd. He has worked in listed and private companies in various industries including retail, wholesale, logistics, construction and public accounting. Stephen specialises in management reporting, system improvement, financial management and mergers and acquisitions. As a member of the executive team he aims to provide enhanced services to the board of directors and shareholders of Allied Healthcare Group. Stephen has a Bachelor of Business majoring in Accounting and Business Law and is a member of the Institute of Chartered Accountants.Lynne Bradshaw - Director-Marketing
Lynne Bradshaw has 25 years experience in senior level management roles and Director positions in the public and private healthcare sector both locally and internationally. Lynne brings to Allied Healthcare Group executive level experience and understanding of pharmaceuticals, medical devices and medical consumables as well as provision of services to the healthcare market. Her career has focused on business development, marketing and distribution in this industry sector. Having established and managed her own medical businesses, Lynne is well equipped to contend with the challenges of a growing a business and has been a trusted consultant to the healthcare industry on major projects. Her wide ranging career experience spans local and international markets where she has built an extensive network of client and industry contacts, developed new products and agencies with international partners and brought innovative medical devices to the Australian market. Lynne is currently Chair and Director of a major Australian charity.Danny Zanardo - Director-Sales
Danny Zanardo has over 20 years experience in the Australian Health Care sector via positions with multinational pharmaceutical companies, biopharmaceutical start-up, and medical device companies. He has gained formal qualifications via a degree in applied science and has built upon this with post graduate business studies, and is currently working towards completing his MBA. During his career Danny has developed strong networks and an intimate understanding of medical device and pharmaceutical commercial and clinical environments. He has a successful track record of leading sales, marketing and clinical teams in commercial projects, with new and existing medical technology, that have resulted in significant revenue growth. Danny has worked with Rousell Uclaf, Hoffman La Roche, and Actelion. He joined Allied Medical as a Regional Sales Manager in 2004 and became National Sales Manager in 2005.
#4 Financial Resources
All information presented in this post has been sourced through publicly available information. In no way or form should my opinion be considered as investment advice. I am currently holding a position in AHZ and make no representation or warranty as to the accuracy of information posted on this blog, and encourage any potential investor of AHZ to follow up by conducting further research with any information which has been obtained from this site for the purpose of forming ones own investment decision - Best of luck with all your investments.




















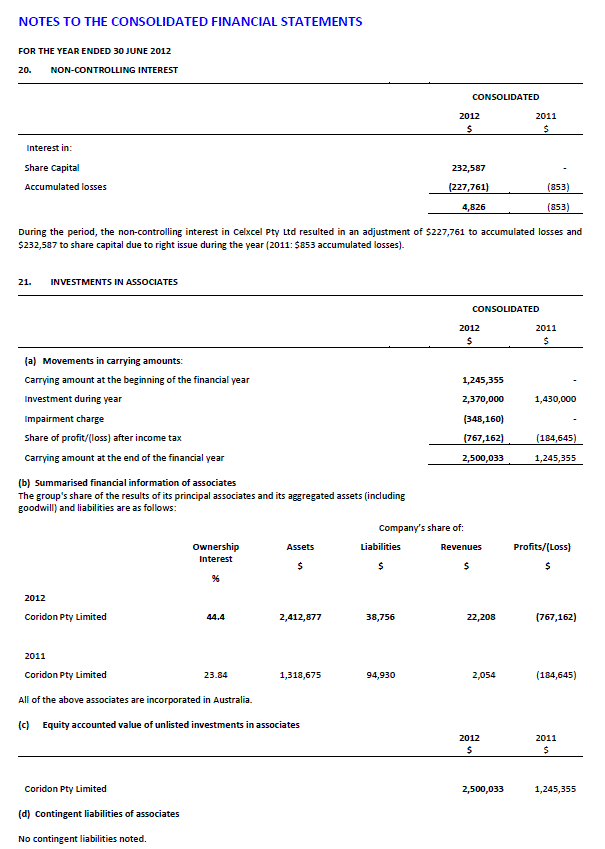










Great work mate. Lex
ReplyDeleteFantastic Lex, a lot of hard work and I thank you kindly. LTL.
ReplyDeleteIrrespective of receiving daily oral or future injectable depot therapies, these require health care visits for medication and monitoring of safety and response. If patients are treated early enough, before a lot of immune system damage has occurred, life expectancy is close to normal, as long as they remain on successful treatment. However, when patients stop therapy, virus rebounds to high levels in most patients, sometimes associated with severe illness because i have gone through this and even an increased risk of death. The aim of “cure”is ongoing but i still do believe my government made millions of ARV drugs instead of finding a cure. for ongoing therapy and monitoring. ARV alone cannot cure HIV as among the cells that are infected are very long-living CD4 memory cells and possibly other cells that act as long-term reservoirs. HIV can hide in these cells without being detected by the body’s immune system. Therefore even when ART completely blocks subsequent rounds of infection of cells, reservoirs that have been infected before therapy initiation persist and from these reservoirs HIV rebounds if therapy is stopped. “Cure” could either mean an eradication cure, which means to completely rid the body of reservoir virus or a functional HIV cure, where HIV may remain in reservoir cells but rebound to high levels is prevented after therapy interruption.Dr Itua Herbal Medicine makes me believes there is a hope for people suffering from,Parkinson's disease,Schizophrenia,Cancer,Scoliosis,Fibromyalgia,Fluoroquinolone Toxicity
ReplyDeleteSyndrome Fibrodysplasia Ossificans Progressiva.Fatal Familial Insomnia Factor V Leiden Mutation ,Epilepsy Dupuytren's disease,Desmoplastic small-round-cell tumor Diabetes ,Coeliac disease,Creutzfeldt–Jakob disease,Cerebral Amyloid Angiopathy, Ataxia,Arthritis,Amyotrophic Lateral Sclerosis,Alzheimer's disease,Adrenocortical carcinoma.Asthma,Allergic diseases.Hiv_ Aids,Herpe ,Copd,Diabetes,Hepatitis,I read about him online how he cure Tasha and Tara so i contacted him on drituaherbalcenter@gmail.com even talked on whatsapps +2348149277967 believe me it was easy i drank his herbal medicine for two weeks and i was cured just like that isn't Dr Itua a wonder man? Yes he is! I thank him so much so i will advise if you are suffering from one of those diseases Pls do contact him he's a nice man.