16/12/13 - GSK
PHARMACEUTICALS PROGRESSES TO SECOND PHASE
OBJ advised that it has
executed a second Analgesic Study Agreement with GlaxoSmithKline (USA) following
the successful completion of the Phase I program. GSK has authorized the
following statement:
"GlaxoSmithKline (USA) has entered into a Phase II Analgesic Study Agreement with OBJ following the successful completion of a Phase 1 program conducted during 2012. The Agreement sets out a development program of the design and optimization of magnetic micro-arrays specifically for GSK proprietary formulations".
In 2012, OBJ and GSK Pharmaceuticals of New Jersey USA, entered into a Phase I Development Agreement designed to determine the effectiveness of OBJ’s Field-in-Motion applicator technology on the ex-vivo transdermal delivery of a number of GSK proprietary analgesic formulations against competitor products. Following completion of the Phase I program, technical discussions commenced on a Phase II Study Agreement and were concluded with GSK’s Analgesics Group now located in Singapore.
"GlaxoSmithKline (USA) has entered into a Phase II Analgesic Study Agreement with OBJ following the successful completion of a Phase 1 program conducted during 2012. The Agreement sets out a development program of the design and optimization of magnetic micro-arrays specifically for GSK proprietary formulations".
In 2012, OBJ and GSK Pharmaceuticals of New Jersey USA, entered into a Phase I Development Agreement designed to determine the effectiveness of OBJ’s Field-in-Motion applicator technology on the ex-vivo transdermal delivery of a number of GSK proprietary analgesic formulations against competitor products. Following completion of the Phase I program, technical discussions commenced on a Phase II Study Agreement and were concluded with GSK’s Analgesics Group now located in Singapore.
13/12/13 - APPOINTMENT OF
LICENSING SPECIALIST
OBJ announced that it has secured the services of Dr. Steve Meller, recently retired Chief Innovation Catalyst at P&G based in Cincinnati to assist in negotiations of partnering and Licensing for the use of OBJ's technologies. Dr. Meller was born and raised in South Australia and obtained his B.Sc., BSc. (Hons.), and PhD from the University of Adelaide. Dr. Meller’s first career was as a research scientist in Neuroscience at the University of Adelaide, The University of Iowa and The University of Cincinnati. Subsequently Dr. Meller joined Procter and Gamble where he has had close to 20 years experience in Senior Health, Innovation and Sustainability leadership roles and worked extensively across 6 continents. Dr. Meller now runs a specialty consulting company from San Francisco and Silicon Valley, works extensively in the US Venture Capital Industry and sits on a number of Advisory Boards. Dr Meller will work closely with OBJ in future negotiations and discussions with Global FMCG and Pharmaceutical companies and advice on further opportunities for OBJ’s technologies.
OBJ announced that it has secured the services of Dr. Steve Meller, recently retired Chief Innovation Catalyst at P&G based in Cincinnati to assist in negotiations of partnering and Licensing for the use of OBJ's technologies. Dr. Meller was born and raised in South Australia and obtained his B.Sc., BSc. (Hons.), and PhD from the University of Adelaide. Dr. Meller’s first career was as a research scientist in Neuroscience at the University of Adelaide, The University of Iowa and The University of Cincinnati. Subsequently Dr. Meller joined Procter and Gamble where he has had close to 20 years experience in Senior Health, Innovation and Sustainability leadership roles and worked extensively across 6 continents. Dr. Meller now runs a specialty consulting company from San Francisco and Silicon Valley, works extensively in the US Venture Capital Industry and sits on a number of Advisory Boards. Dr Meller will work closely with OBJ in future negotiations and discussions with Global FMCG and Pharmaceutical companies and advice on further opportunities for OBJ’s technologies.
13/12/13 - FIRST BODYGUARD
TRIAL SUCCESS
OBJ advised that the first product trial of its BodyGuard Knee patch has
exceeded expectations and improved knee functionality in 94% of volunteers in a
two week, Aggregated Locomotor Function (ALF) study. The study, designed by
Professor Tony Wright, combined 6 knee intensive challenges. The program was
developed to evaluate the effect of the Bodyguard patch technology and OBJ’s
Lubricen formulation on healthy male volunteers following two weeks of daily use
of the BodyGuard patch. Improvement in performance in the timed challenges of
the ALF program resulted in 16 out of 17 participants showing substantial
improvements in their aggregated times. The distance challenges resulted in
substantial gains in 12 of the 17 participants over the two week treatment
period. The overall performance improvement following two weeks of patch use
across all participants and all challenges was 14% which Professor Wright has
indicated as being clinically important.
Improving knee function is a major unmet medical need with incidence of age and injury related joint deficiency now affecting 1 in 5 people in the developed world. Loss of knee function through injury, overuse or degeneration is known to increase the likelihood of Osteoarthritis. The goal of the BodyGuard project is to develop a new form of transdermal patch that can hydrate and lubricate the articular surfaces of joints, potentially reversing the effect of joint ageing and degeneration. In addition to the ALF program, the study also utilised thermal imaging before and after exercise to determine whether the patch altered localised tissue trauma during intensive exercise. A Knee Injury/Osteoarthritis Outcome Score (KOOS) questionnaire and a daily participant diary were also part of the study and will be used to determine participant perceptions and usage experiences, all of which will be used to further refine the next phase of trials.
As a result of the success of this first trial, the Company plans to undertake a larger independent clinical trial during 2014. A number of Australian and international scientific and sporting clinical centres are under consideration. The outcomes of such a clinical trial would then underpin the Company’s business planning, partnering and distribution discussions.
BodyGuard was established by OBJ to utilise its low cost magnetic microarray drug delivery technology in a new form of transdermal patch designed to limit joint ageing and to restore healthy joint function. By combining OBJ’s drug delivery technology and Lubricen formulation with the patch’s physical kinesiology, proprioception and tendon stabilisation properties, a unique and highly advanced joint treatment product platform will be created. The product would create a new and potentially unique category for the treatment of joint injury, joint ageing and osteoarthritis.
The Company plans to commercialise the BodyGuard range of products through distribution and branded supply relationships with established global brand leaders across the sporting, life-style and osteoarthritis sectors. The Company is currently exploring new patch designs for other major joints of the body and new formulations for performance enhancement, injury recovery and pain management.
Improving knee function is a major unmet medical need with incidence of age and injury related joint deficiency now affecting 1 in 5 people in the developed world. Loss of knee function through injury, overuse or degeneration is known to increase the likelihood of Osteoarthritis. The goal of the BodyGuard project is to develop a new form of transdermal patch that can hydrate and lubricate the articular surfaces of joints, potentially reversing the effect of joint ageing and degeneration. In addition to the ALF program, the study also utilised thermal imaging before and after exercise to determine whether the patch altered localised tissue trauma during intensive exercise. A Knee Injury/Osteoarthritis Outcome Score (KOOS) questionnaire and a daily participant diary were also part of the study and will be used to determine participant perceptions and usage experiences, all of which will be used to further refine the next phase of trials.
As a result of the success of this first trial, the Company plans to undertake a larger independent clinical trial during 2014. A number of Australian and international scientific and sporting clinical centres are under consideration. The outcomes of such a clinical trial would then underpin the Company’s business planning, partnering and distribution discussions.
BodyGuard was established by OBJ to utilise its low cost magnetic microarray drug delivery technology in a new form of transdermal patch designed to limit joint ageing and to restore healthy joint function. By combining OBJ’s drug delivery technology and Lubricen formulation with the patch’s physical kinesiology, proprioception and tendon stabilisation properties, a unique and highly advanced joint treatment product platform will be created. The product would create a new and potentially unique category for the treatment of joint injury, joint ageing and osteoarthritis.
The Company plans to commercialise the BodyGuard range of products through distribution and branded supply relationships with established global brand leaders across the sporting, life-style and osteoarthritis sectors. The Company is currently exploring new patch designs for other major joints of the body and new formulations for performance enhancement, injury recovery and pain management.
AGM Presentation Transcript - Jeff Edwards
We are involved in a lot of projects, we could spend hours on each one and
product development and technology platform, etc. However, I have prepared
a highlight presentation so you really need to focus down on the key aspects
on what the company has been doing in the last year and from that you should be
able to project what is going to happen in the future.
The big issue for
OBJ, or where we find ourselves this year up until 2013, we were really a
technology development company in many aspects, we were working with partners to
get an understanding of how our technology platforms could be incorporated into
products and provide commercially relevant solutions for them, thus generating
revenues. 2013 has really seen quite a significant shift from what is
effectively R&D, into working with our Partners to develop solutions. And
these are market focused solutions - we have gone beyond the technology platforms
and we are now in the solutions business, hand in hand with some of the largest
companies in the world. We still remain absolutely focused in the cosmetic &
skincare area - these are clearly areas that we can bring a great deal of value
add, a lot of our commercial business is focused on that. Consumer Health care
is another area that we are focused on because we now have the ability to create
solutions to problems rather than technology alone - we have grown our strength
and our capability in that area through the year and in Oral Healthcare - Oral
Healthcare started approximately 3 years ago, I suppose as a very broad concept
of, “wow wouldn’t it be great if” - we’ve now gone beyond all of that - we have
covered so many ex vivo and first in human studies and as we’ve announced there is a
major human clinical study to be conducted in the UK, just showing how much
progress we’ve made on Oral Healthcare - we will touch on that in a
minute.
Pharmaceuticals
Where we are still keeping
relatively away from long term deeply expensive pharmaceuticals activities we
quite like what’s with OTC (Over-the-counter) - OTC short gestation period large
volume, the big end of town in the very heavy weight pharmaceuticals is still an
area that we will hold back on in preference to rapid to market higher turnover
consumer based products. So that’s really where we are it’s really absolutely
consistent with the story we gave last year and the year before and in fact
there is an absolute sequential program that we have moved through where we have
achieved every one of the goals that we have set ourselves with every one of our
clients. I will now touch on each of those I think will be interesting.
Proctor & Gamble
Clearly P&G are foremost on every
one’s mind. P&G are the world’s largest consumer company, you virtually
cannot open your kitchen cupboard or your bathroom cupboard without being
inundated with P&G products they have over 2000 of the world’s biggest
selling sku’s. Our relationship with P&G is in an exclusive area within
their Beauty & Grooming group and that is divided into 7 sub categories and
we’re working with those - skincare clearly being the foremost one that we’re
moving forward with. We have completed the first of 3 clinical studies and in
fact the most important of those with P&G. Before you ask, no I can’t tell
you what the outcomes of those are because the clinical results are not OBJ’s
property, however the outcomes are being sufficiently engaging for them that we
are now moving - that we have now moved forward into negotiations regarding
possible product development for the first application of OBJ’s technologies
across P&G’s product range. I hope to be able to expand on that in coming
announcements shortly.
Coty – philosophy
The next one that
was quite recently was the Coty – philosophy program, the philosophy clinical
program that was a very large one in the U.S is now complete, again the results
are not ours, I can’t share it with you, however I think that you can clearly
see that the results have been sufficiently engaging and encouraging that we are
now have moved forward and philosophy and OBJ are currently in negotiations for
a development program which will lead on to a licensing agreement, from there,
relatively advanced on those sections, so those 2 major clinical events that we
announced earlier in the year have achieved everything that OBJ wished to and
have been sufficiently encouraging that we are now engaged with these major
parties on how you now turn those into products.
Oral
Healthcare
As I have just mentioned earlier your Oral Health, Oral
Health is potentially a very big area for us because the technology lends its
self so easily to the toothbrush business there have been no major changes in
toothbrush, manual toothbrush technology in probably the last 40 years this will
be the very first, we have done multiple ex vivo studies, we have done first in
mouth pilot studies, all of those have been encouraging the whole length and
have encouraged GSK to commit to a rather large human clinical [?] study, as
Glyn said we had expected that to commenced by now, but there are hold ups with
the English ethics groups but they are just temporary things we expect to go on
that any day now. We are very enthusiastic about this space it’s really where
OBJ’s technology can change people’s lives we may have mentioned before, 1%
improvement in fluoride uptake in teeth will save 1,000,000 children’s teeth in
Australia alone so we think the level of enhancement we get starts to make
serious social impact.
GSK Pharmaceuticals
GSK
Pharmaceuticals, this is a development that has moved quite rapidly for us it is
an OTC pharmaceutical area we previously announced that we were working with
them on a phase 1 optimisation program where we are applying OBJ’s technology to
an existing GSK formulation, they were having some trouble competing in the
market with the performance of their product so they came to OBJ, we have now
completed that phase 1 program and that is now moving forward to negotiations
and expansion into a much larger phase 2 development program with GSK. A very
convenient one because GSK is moving that division out of the U.S and into south
east Asia much closer to our home ground, it means that I won’t have to spend
quite so much time on aeroplanes in the future which I think is great. So that
is another one area that we are moving very, very forward
with.
BodyGuard Program
Our own products the BodyGuard,
George will be doing a separate presentation on this but really 2013 has seen a
massive movement forward on that we are very excited about that one I think that
you will share that enthusiasm once George has finished, I will try not to steal
any of his thunder, but this is very serious clinical program under the
supervision of a guy, Professor Tony Wright, we are making this a world class
event and George will tell you exactly where we are and why we are so excited
about those thing’s.
Other Events
Other events during the
year, there the primary ones that everybody’s mind was on, Coty, P&G, GSK,
GSK & our own product. Just other things so that you can get a sense of how
we have grown through the year we have now appointed a new Operations Manager,
now that we have moved into the solutions business essential that we have the
resources, the capability the in-house to be able to satisfy our partner’s
manufacturing capabilities, spent most of last night on a phone call to an
overseas company trying to work out things, logistics of producing products with
OBJ’s technology so we are seriously growing our strengths in these commercial
steps areas. Again we have really grown our test models and expertise because of
our ability to influence partners, something that we learnt I suppose, last year
as we were allowed deeper and deeper into these multi-billion dollar companies
we really did realise that OBJ’s technical strength was actually quite
significantly superior to their own and these are the biggest companies in the
world at this stage, we are now being asked to support those development
programs, much stronger, because we can offer a speed of results and a
capability that’s beyond the vast majority of our partners now, so we moved not
only the technology, but the development steps, all chargeable development steps
so we can turn our lab from a research centre into a profit centre so we have
grown that strength and capability through the year quite extensively. But
in-spite of all those things if you look at our expenditure we have been able to
maintain that irrespective of our growth we are still considered one of the most
conservative expenditure companies, we are very careful about what we do with
your money because we believe that it is a valuable and finite resource, we
waste nothing.
And of course the recent capital raising with Dirk (Baker
& Young) and the help of our loyal supporters have now really put the
company in a position where it has vision, capital resources to now really drive
these areas home, especially with such exciting projects as George has a level
of support, where getting from our partners again keeping in mind that all of
our partner activities are fully funded, yet they put up the cheques. The days
of us investing in partner activities are gone - it’s now joint funded they're
paying the bills and that really changes the economics, dynamics so we can focus
on how we turn that into strong and sustainable cash flow, so we can grow the
value that under pins the work of OBJ. So in a nutshell, that’s what we have
done with your company during 2013, it’s been a terribly exciting period and I
think there is some very near term stuff that I think that you will be very
happy with that we will be very very keen to announce as soon as we possibly
can.
September 2013
Annual Report
Letter to shareholders
Letter to shareholders
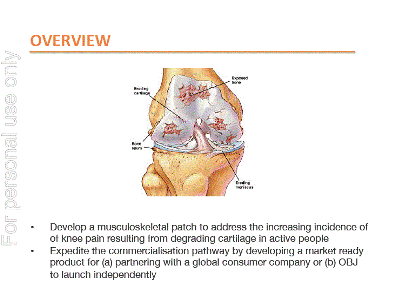
During the period, the Company extended its relationships with its international partnering companies that led to the first human clinical studies being undertaken utilizing OBJ’s technologies. This was the culmination of many years of consistent hard work in nurturing relationships, undertaking numerous efficacy tests and broadening potential applications. The Company has also chosen the musculoskeletal and pain patch sector as its first product offering. The development of the Company’s own product lines for application to the knee is progressing well with a design firm now undertaking design work for the patch in preparation for testing. It is the intention of the Company to undertake the development of its own series of products but to utilize established distribution partners and brands for marketing.
During the year, OBJ executed an agreement with Procter & Gamble (P&G) to undertake human clinical studies with three P&G product applications. These series of clinical studies are undertaken for establishing the claims to be available to Procter and Gamble in the event that OBJ’s technologies are incorporated into P&G’s future product lines. In addition, during the year the Company announced testing of applications for the dermaportation technology with Coty cosmetic applications. The Company also gained patent protection for dermaportation which is being evaluated by Coty in both the European and US regions. The Company also reported during the year continuing successes in the Oral Health field in its exclusive collaboration with GlaxoSmithKline (GSK) in the caries (tooth decay) field. The Company has agreed with GSK to undertake a series of human trials in the UK using OBJ’s technology in oral health applications. Cosmetics and skin care remain a key focus for the Company as it provides a broad set of market applications for products that incorporate OBJ’s technologies and likely to be a shorter route to market than the traditional pharmaceutical market sector. The Company maintains its development program across many pharmaceutical applications with targeted partnering firms.
OBJ is moving forward with the testing and development of specific market applications for its technologies as it moves from being principally a technology R&D entity to a product development company. At the same time, work is well advanced with the Company’s own musculoskeletal and pain patch development that should see OBJ for the first time able to reach its market under its own timetable.
I would like to take this opportunity of thanking our shareholders in supporting the Company as we transition OBJ into a more development orientated business. A great deal has been achieved this year in that transition phase. I would like to particularly thank my fellow Directors for their regular discussions and advice over many years.
Review of Operations
The 2013 financial year has been one of consolidation and progress for the Company on many fronts as its technologies are increasingly being accepted by global brand leaders as the next generation in product innovation. OBJ’s technologies are now at the fore front of new product developments across a number of main-stream commercial sectors and with a number of the world’s leading companies.
The transition from Research and Development to Product Innovation has been an exciting and rewarding journey for all areas of the Company. The Company’s focus on Consumer Healthcare, Oral Health, Skin Care and its own internal developments in joint pain management has yielded positive progress. During the period, all of the major reported projects have progressed and the Directors are pleased to provide the following summary of partnering activities together with our own product development, marketing initiatives and technical developments.
While the Company has a number of international partnering programs in various stages, the relationships with Procter & Gamble (P&G), GlaxoSmithKline (GSK) and Coty are particularly important initiatives.
In 2012, the Company announced it had executed an exclusive Joint Development Agreement (JDA) with P&G, the world’s largest consumer products company. During the 2013 financial year, this collaboration led to the development of a number of custom magnetic micro-arrays designed to enhance the time to onset and level of performance of several key P&G ingredients used across multiple consumer products. Early successes in evaluation programs encouraged a rapid move to the design and development of consumer relevant prototypes for clinical trialing. These study programs are progressing and are expected to conclude by the end of 2013.
GLAXOSMITHKLINE CONSUMER HEALTHCARE - ORAL HEALTH
| In early 2012, the Company reported continuing success in the enhanced delivery of active ingredients employed by the oral healthcare industry in a joint development program with GSK Consumer Healthcare Group in the UK. Early successes encouraged GSK to fund the first in-mouth human pilot study which was conducted in early 2013. As a result o f these early studies, the focus has moved to the development of new toothbrush technology incorporating OBJ’s technologies that could facilitate broader clinical evaluations. The Board is pleased to report that this challenging program has bee n successfully completed by the Company’s R&D group and that GSK and OBJ will now move forward into a clinical study using OBJ’s technology. This program will initially explore enhanced fluoride delivery using OBJ’s technology. There are also two additional key areas where OBJ’s FIM technology has been previously shown to be effective. . GSK PHARMACEUTICALS (USA) - TOPICAL ANALGESICS The Company’s early work with GSK Analgesics in the USA continued during the period with the successful completion of a PHASE I study program to explore the impact on delivery of the OBJ technology on a number of proprietary formulations developed by GSK. Discussions and negotiations for a PHASE II optimization program are currently at the planning stage. The image (right) shows the Company’s “Super Tube” measured dose applicator which is well suite d to the controlled application of various therapeutic products and is under consideration by a number of potential partnering companies. COTY - PHILOSOPHY - PRESTIGE SKINCARE During the period, the Company announced its collaboration with the Coty Group’s prestige cosmetic skincare company Philosophy. That collaboration is based around the Company’s powered technology and the e-Skin platform that includes an e-commerce component. Progress with Coty has been rapid with the Company designing and manufacturing a customer skincare device for clinical trialing and COTY commissioning a third-party contract research organization to conduct a clinical study.
Early indicative results have been encouraging and the Company is now working with Coty to develop a new “Wand” format incorporating Coty’s desired commercial attributes. A number of other parties have also expressed an interest in various products based on the powered and e-Skin technology platforms. OBJ’s PRODUCTS GROUP REPORT (Mr George Tsadilas) BodyGuard The Company continues with the development of a m usculoskeletal patch with the appointment of a fu ll time Manager to facilitate the design, testing and roll-out of the BodyGuard range of products. A thorough market appraisal has been conducted of the first product for the application to the knee and a business model has been developed for implementation. T he appraisal indicated that a significant global opportunity exists for a knee patch in three target segments: • Athletes; • Middle aged active people; and • Osteoarthritis sufferers. A design house has been engaged to design and develop a number of product prototypes in order to conduct product efficacy testing, clinical trials and consumer research. OBJ’s laboratory has been responsible for the formulation development in conjunction with the London School of Pharmacy which has successfully shown enhanced delivery of key aggrecan at rates greater than leading European brands. The intention is for the Company to develop a full commercial offering package before seeking branded companies to distribute the product. INTERNATIONAL PARTNERING REPORT (Dr Kevin Hammond) The past twelve months has seen the Company move into a far more dynamic phase in partnering activities. Earlier efforts focused on consolidating collaborations, extending the technology portfolio, and delivering proof of principle data. These have now culminated in significant developments with partners in the FMCG Cosmetic and Personal Care sectors. Two key partners, P&G and Coty, have committed to clinical evaluation studies. As recently announced, GSK has now committed to significant clinical studies in the oral health care sector. OBJ is working closely and continuously alongside partners in all these studies. These commitments to undertake clinical trials are very positive intentions to consider OBJ’s technologies across a variety of applications. Partnering activities in the pharmaceutical sector have also continued positively. Safety and regulatory factors inevitably necessitate a cautious pace. Internally, significant progress has also been made on the BodyGuard program. This has not detracted from our ongoing partnering activities. OBJ identified a gap in the musculoskeletal injuries market and the opportunity to exploit it with its unique technology. TECHNOLOGY DEVELOPMENT REPORT (Dr Matthew Mclldowie) The OBJ technical team has had a successful year. The primary focus has been the evolution of OBJ’s Dennaportation and FIM technology platforms into consumer focused commercial products. The high quality data produced in the laboratory for the P&G “Proof of Principle” assessment program of in vitro and in vivo work in 2012 has resulted in the advancement of the collaboration to the clinical trial stage. Design and development of the clinical trial units was undertaken in-house, demonstrating OBJ’s ability to provide expertise from technology development through to pre-production in support of partnering clients. In response to the need for rapid development of production quality Dermaportation and FIM based units, OBJ acquired in-house 3D modeling and 3D printing capabilities. This additional capability has accelerated the design process significantly and allowed OBJ to quickly tailor devices to the specifications that FMCG partners require. An extensive program of formulation design and testing in support of the BodyGuard series of products was initiated in early 2013. This has already resulted in significant positive data obtained in vitro with regard to the through-skin delivery of OBJ’s Lubricen™ formulation. The formulation testing program is ongoing, and OBJ looks forward to further enhancements being achieved with the cooperation of its Australian design partners. 2013 Annual Report |
August 2013
Patent published for WO2013110124
Nature Journal - Transdermal Drug Delivery Facilitated Using A Combination of Microneedles and A Magnetophoretic Patch
July 2013
GSK Oral Healthcare Moves to Clinical Evaluation
OBJ Limited announced that it has executed a Clinical Evaluation and Exclusivity Agreement with GlaxoSmithKline (“GSK”) Consumer Healthcare in preparation for a human clinical trial in the field of Oral Healthcare. Under the Agreement OBJ has granted GSK an exclusive right to conduct the study and to negotiate future licensing terms and conditions in consideration for GSK funding the clinical trial plus OBJ’s costs and expenses in supporting the trial. The study will be the largest undertaken in the area of oral healthcare using OBJ’s FIM™ technology.
GSK has approved the following statement:
“OBJ and GSK have executed a Clinical Evaluation and Exclusivity Agreement which contains the terms and conditions under which OBJ and GSK have agreed to work exclusively together for GSK to evaluate OBJ’s FIM microarray technology with the objective of performing an in vivo study in humans and once the results of the study become available, GSK will discuss with OBJ potential further agreements for the development of OBJ’s proprietary technologies in the field of oral
healthcare. GSK will conduct the human study and OBJ will support GSK by providing the OBJ Technology and technical support as requested by GSK from time to time

The Clinical Evaluation
The clinical evaluation will be conducted in the UK by GSK’s Oral Healthcare group in Middlesex. The study is expected to commence towards the end of 2013 however the duration, number of subjects and the detailed protocols to be employed in the study are still being planned in preparation of submission for ethical approval.
GSK has approved the following statement:
“OBJ and GSK have executed a Clinical Evaluation and Exclusivity Agreement which contains the terms and conditions under which OBJ and GSK have agreed to work exclusively together for GSK to evaluate OBJ’s FIM microarray technology with the objective of performing an in vivo study in humans and once the results of the study become available, GSK will discuss with OBJ potential further agreements for the development of OBJ’s proprietary technologies in the field of oral
healthcare. GSK will conduct the human study and OBJ will support GSK by providing the OBJ Technology and technical support as requested by GSK from time to time

The Clinical Evaluation
The clinical evaluation will be conducted in the UK by GSK’s Oral Healthcare group in Middlesex. The study is expected to commence towards the end of 2013 however the duration, number of subjects and the detailed protocols to be employed in the study are still being planned in preparation of submission for ethical approval.
Next Generation Toothbrush
OBJ’s proprietary thin‐film magnetic micro‐arrays are well suited to oral health and toothbrush production as the low cost and flexible design is highly compatible with modern toothbrush production line technologies. The ability to integrate drug delivery technology into a toothbrush at very low additional cost, resulting in a new approach to improved oral hygiene has the potential to go beyond the scope of toothpaste chemistry alone. The low cost, high performance capabilities of OBJ’s toothbrush technology is especially applicable to new growth markets.
June 2013
May 2013
BodyGuard Program Update
OBJ Limited reported that its musculoskeletal patch development, known internally as BodyGuard, has achieved several significant milestones in its ongoing development program
Planning for the overall BodyGuard program is progressing with the first steps in the industrial
design and formulation optimisation now well advanced. Further assessment of the positioning of the product in its selected market sector has been completed and reconfirms the initial positioning selected. The Company is not aware of any active patch in the market today that is able to provide the benefits offered by the BodyGuard series application. It is intended that the BodyGuard series of products will ultimately be designed for application to various body joints.
OBJ’s Product Development Manager for the overall management of BodyGuard products was appointed to the position during the last quarter of 2012.
International Design
In late 2012 the Company invited several major international design houses with sports and
medical design experience to tender for the design and prototype production of BodyGuard’s first family of products for the treatment of various joint, ligament, tendon and cartilage injuries and
degenerative conditions. The selected tenderer will be required to prepare alternative designs for
the first product, material selection and testing and prepare a number of prototype products that
can then be used in forthcoming clinical trials. A shortlist of candidate companies was prepared
from the excellent proposals received. During March, the leading design companies were
interviewed by our Product Development Manager and OBJ is now evaluating the recommendations to progress this key activity. The successful design house will be responsible for the key elements relating to look, feel and manufacturing form for the first family of BodyGuard products.
Formulation
In late 2012 the Company invited several major international design houses with sports and
medical design experience to tender for the design and prototype production of BodyGuard’s first family of products for the treatment of various joint, ligament, tendon and cartilage injuries and
degenerative conditions. The selected tenderer will be required to prepare alternative designs for
the first product, material selection and testing and prepare a number of prototype products that
can then be used in forthcoming clinical trials. A shortlist of candidate companies was prepared
from the excellent proposals received. During March, the leading design companies were
interviewed by our Product Development Manager and OBJ is now evaluating the recommendations to progress this key activity. The successful design house will be responsible for the key elements relating to look, feel and manufacturing form for the first family of BodyGuard products.
Formulation
Recent in vitro human skin penetration studies of OBJ’s Lubricen™ formulation, in conjunction with the new specially designed magnetic microarray, achieved through‐the‐skin delivery rates significantly greater than Europe’s leading topical joint pain product OBJ’s proprietary Lubricen™
formulation, which was designed to increase joint, tendon and ligament lubrication by mimicking
natural synovial fluid aggrecans, was developed by OBJ’s scientific team in conjunction with
Professor Adrian Davis of the London School of Pharmacy, one of the leading topical formulation
specialists.

Independent research has confirmed the widespread discomfort experienced by many people
who undertake regular exercise and by people who suffer osteoporosis particularly as they get
older. The Bodyguard active patches are intended to provide localized relief to patients rather
than the commonly used systemic applications today.
Clinical Testing Programs
Clinical testing of the BodyGuard patches to demonstrate functional capabilities and superior
performance is currently being planned through a number of leading sport science, exercise and
health campuses in both Australia and Europe. Clinical testing will commence following the
completion of pre‐production prototype development.
Distribution
The Company will undertake this development program bringing each of the BodyGuard applications to a production stage by selecting the most efficient method of distribution through established brands or distributing the products itself.
ESKIN CLINICAL TRIAL UNITS SHIPPED TO THE USA
OBJ Limited (ASX:OBJ) is pleased to announce that it has manufactured and shipped the first clinical trial eSkin™ units to its partner’s Contract Research Organization (CRO) in the USA. The shipment is part of the Exclusive Collaboration Agreement involving the Company’s proprietary eSkin™ technology, announced to the market on 25th February 2013. The clinical trial units were developed by the Company in consultation with its partner’s scientific teams in the USA and Europe and will be evaluated in an independent clinical efficacy study in the USA.

The clinical trial will serve to prove up efficacy in humans for marketing claims and, subject to success in the clinical trial, as a pre-cursor to potential licensing, supply and global distribution.
eSkin™ incorporates OBJ’s patented Dermaportation powered transdermal delivery technology that has been shown to enhance the delivery of key anti‐aging and skincare ingredients deeper and more effectively into the skin. The clinical trial is designed to demonstrate consumer perceivable levels of performance improvement including more rapid reduction of fine lines and wrinkles in comparison with the commercial formulation applied by hand. The use of an independent CRO validates the
data generated to then be potentially used commercially throughout the world. If successful the clinical trial will be a major step forward for the Company in the potential commercial development of its powered drug delivery technology and its web‐enabled personalisation capabilities that allow eSkin™ to optimise cosmetic delivery precisely to individual needs via the world wide web using portable devices.
March 2013
GRANTING OF EUROPEAN PATENT FOR DERMAPORTATION TECHNOLOGY
Following the allowance claims announced by OBJ Limited on 10 December 2012 the European Patent Office have issued a key patent for OBJ's powered Dermaportation technology which is expected to allow the company to offer various market licensing options to global partners in key market sectors.
The US Patent Office granted this same patent to OBJ in November of 2011.
Dermaportation was the Company’s first drug delivery technology and has recently been incorporated into the e‐Skin® cosmetic device system which is the subject of commercial discussions with a global skin care company
Dermaportation Technology
The technology generates a series of repeating quasi-rectangular waves of electromagnetic energy with peak maximum field strength of between 5 mT (milli Tesla) by a secure microprocessor smart technology with automatic Cyclic Redundancy Check (CRC) data integrity testing and systems integrity testing to ensure the quality and repeatability of the field characteristics. Designed for applications where repeat applications, extended usage and multi-formulation requirements make a powered reusable device the best solution. Able to be implemented as a power-patch, a wand, an instrument or an iPod-like device. Dermaportation is well suited to many professional and retail applications where repeat delivery of consumables or the targeted delivery of actives is beneficial.
LIPID AGGREGATION
The Dermaportation technique uses highly controlled patterns of low voltage energy. Rather than destroying the bilayer’s underlying structure, it is proposed that Dermaportation creates temporary constrainments within the lipid bilayers. While the precise methods used to achieve this are a matter of commercial confidentiality, this allows Dermaportation to create temporary regions of low probability densities, which manifest as ring-like voids surrounding a compacted lipid core, through which therapeutic drugs and compounds can readily pass. The Dermaportation technique is an analogue process where the size, sequence and duration of the generated voids are all controllable.
INDUCING THE DERMAPORTATION EFFECT
The Dermaportation process uses an induction technique as the mechanism for manipulating the stratum corneum lipid bilayers. This technique has a number of important practical and clinical benefits.
TARGETING
The inductive technique used allows Dermaportation to target accurately within 3 dimensions with no need for sophisticated targeting systems. The physical location of the inductive head in relation to the skin allows accurate and defined areas of action while frequency control allows Dermaportation to target the stratum corneum bilayers only, with little or no detectable effect in surrounding tissues.
CONTROL
Dermaportation provides a number of control functions, including void creation, void closure, induced compound mobilization and controlled release. This allows Dermaportation to be used as a drug delivery protocol rather than a single process.
LOW ENERGY
The amount of energy produced by the Dermaportation system is approximately 1,000 times smaller than the stratum corneum lipid disruptive processes current used. This makes Dermaportation ideally suited to portability, out-patient and homecare usage.
NON-CONTACT
Dermaportation can operate through most non-conductive materials, including bandages, pads and cloths and functions without the requirement for physical contact. This avoids the traditional problems of allergies and burns often associated with contact pads.
PAINLESS
Dermaportation operates using a modality for which humans have no receptors. As a result, the effect is not detectible by the human body and is totally pain free and comfortable to use.
REGULATORY
Inductive processes have been used clinically throughout the world for over 40 years and are currently approved for human use in all major markets, including USA and Europe. The FDA approved the first inductive system in 1979.
CONCLUSION
Dermaportation is a Drug Delivery technology that provides an unprecedented level of dermal permeability control. The technology is powerful, yet gentle and represents the first of a new generation of bio-communication delivery systems. As such the market applications are enormous and the value of the technology to drug companies, medical professionals and patients/consumers will be considerable.
Dermaportation -
Case
Applications
PAIN
MANAGEMENT
The Intelligent Fentanyl Patch – Patient and computer
controlled transdermal patch
Fentanyl is a powerful
pain management drug, often used for postoperative pain in adults following
major surgery. Current transdermal patches can maintain a steady level of drug
over 72 hours, however if a patient experiences a sudden pain episode, there is
no way to override these with simple patches. Dermaportation will offer
intelligent patches, programmed to accommodate such very real demands but still
function within doctor prescribed parameters. It will record patient usage to be
programmable in the field and recorded for later examination. Intensive care
with the freedom of home care. The capability to add sophisticated controls to
the use of the transdermal patch will substantially increase the effectiveness
of the delivery of pain management drugs and will allow the development of new
drugs amenable to the new delivery parameters.
CHILDREN AS
PATIENTS
Local Anaesthetic - Rapid drug
delivery
Local anaesthetic gels
and creams can take hours to be effective. Such products are commonly used in
children to make injections and venepuncture more tolerable and to lessen the
fear of injections. Emotional upset is common in children facing the prospect of
an injection. Hastening the effect of local anaesthetic and other slow-to-act
transdermal preparations has the potential to streamline a number of clinical
processes and reduce the fear and upset for many pediatric
patients.
Dermaportation has widespread application for the treatment of children.
Dermaportation has widespread application for the treatment of children.
TREATMENT OF
SCARS
Surgery – Scar reduction and
resolution
Scars both new and old
are proving to be responsive to a number of therapies and processes designed to
increase hydration. However, penetrating the scar tissues with therapeutic
agents has been a major hurdle to the broad adoption of such therapies.
Dermaportation has the properties and the molecular manipulation capabilities to
hasten and enhance a number of acute and chronic scar reduction therapies.
Dermaportation is uniquely suited to scar reduction as its small size and ease
of use make it ideal for home-based and extended therapy scar resolution
programs.
SKIN CANCER
TREATMENT
Photodynamic Therapy – Rapid and effective
uptake
Aminolaevulinic acid or
ALA is currently gaining broad support for the non-invasive treatment of skin
cancers (specifically superficial basal cell carcinoma) without scarring. The
drug is applied to the skin (topically) for between 2 and 5 hours and is taken
up by the lesion, which is then irradiated with light at 615nm for ten minutes.
The ALA reacts to the light, changes its structure and destroys the cancer.
Using Dermaportation, the time period for drug application would be shortened
significantly and when combining with lignocaine the pain experienced by
patients would also be eradicated. This overcomes both major disadvantages of
this emerging treatment. Skin cancer rates are increasing with the damage to the
earth’s ozone layer. The potential for a better and more rapid method of
treatment promises to be a major area of opportunity for
Dermaportation.
COSMETIC
RESTORATION
Cosmetics – a new world of dermal
restorative compounds.
The cosmetic world has
been forced to rely on a relatively small number of simple and low molecular
weight compounds in retail formulations, as the majority of more complex and
larger compounds suffer from low absorption through the skin. Dermaportation is
uniquely suited to the needs of both the retail and professional cosmetic
industries. It creates temporary pathways through which these more active
compounds can be introduced, without irritation or damage to the skin’s delicate
structures. Many of the important matrix molecules, which once gave skin its
youthful appearance are subject to sun damage, oxidation and biological
degradation. Dermaportation will enable delivery of these compounds that induce
important biological changes such as new collagen synthesis, manufacture of
ground substance, angiogenesis, and thickening of the epidermis. By allowing
these important compounds to be administered through-the-skin, Dermaportation
will assist in the restoration of damaged skin to an improved state. The global
market for improved cosmetic compound application is possibly the largest
untapped market of all.
The advent of advanced drug
delivery devices has arrived, raising the bar for many traditional devices. The
companies that recognise this fact, and which focus their innovation accordingly
over the next 10 years, will be rewarded with a growing market share by 2020,
leading what will become a highly competitive and very lucrative
industry.
- Source
February 2013
OBJ Limited lands exclusive deal with US beauty giant Coty Inc.
OBJ Ltd Enters Exclusive Collaboration Agreement With COTY Inc.
OBJ Limited lands exclusive partnership with Coty Inc.
AN ENTREPRENEURIAL CULTURE OF INNOVATION
Coty was founded in Paris in 1904 by François Coty, a visionary in the perfume industry.
Today, Coty is a new emerging leader in beauty under the creative, entrepreneurial and visionary leadership of Chief Executive Officer Michele Scannavini.
With about 12,000 employees and corporate headquarters in New York, we still honor François Coty’s mission to “offer a product of rich appearance that is affordable at a variety of price points,” by offering products from ultra-premium luxury to entertainment/lifestyle and accessible price points to match the lifestyle of our consumers.
Our unique portfolio of widely known brands in fragrance, color cosmetics (including nail), plus skin and body care supports net revenues of $4.6 billion for the fiscal year ended June 30, 2012.
Coty’s entrepreneurial culture is driven by a spirit of “FASTER. FURTHER. FREER” that gives us the agility to make fast decisions, push boundaries and support creativity. An example of our company embodying this philosophy is the rapid decision-making process
Appendix 4D - Half Year Operation Update
Review of Operations
The following provides an update on the technical and commercial progress of the Company for the half- year ended 31 December 2012.
The major focus for the period involved the progression of a number of the Company’s International Partnering relationships from technology development and evaluation to consumer focused clinical efficacy trials.
The Company’s developments in the musculoskeletal patch area progressed with continued strong consumer research results and further refinement of the Company’s propriety formulations technologies.
Highlights
Proctor & Gamble
During the period, The Procter and Gamble Company (P&G) committed to two separate human clinical trials following successful pre-clinical developments conducted as part of the Joint Development Agreement (JDA). A number of consumer focused devices containing the OBJ FIM technology were developed and fabricated in preparation for the consumer efficacy studies to be conducted by P&G during 2013.
GlaxoSmithKline
During the period, the Company’s work with GlaxoSmithKline progressed with the successful completion of a number of evaluations and developments in the Oral Healthcare field.
International Collaborations
International collaborations during the period included the successful completion of initial evaluations for the delivery of a widely used topical analgesic compound on behalf of a major international pharmaceutical company. Discussions with a global Top 10 Cosmetic company continued. Those discussions have now advanced to programs of evaluation using the Company’s proprietary eSkin delivery technology.
Appendix 4D Half Year Consolidated Financial Report